Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy
- PMID: 26497992
- PMCID: PMC4662699
- DOI: 10.1212/WNL.0000000000002147
Histologic muscular history in steroid-treated and untreated patients with Duchenne dystrophy
Abstract
Objective: Duchenne muscular dystrophy (DMD) is a lethal disease. The outcome measures used in numerous therapeutic trials include skeletal muscle biopsy. We studied the natural history of DMD from the standpoint of muscle histology with the aim of providing a reproducible tool for use in evaluating and comparing any histologic changes occurring in patients with DMD undergoing treatment and hence be able to determine how therapy modulates the histologic evolution of the disease.
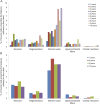
Methods: Three independent operators analyzed 56 muscle biopsies from 40 patients not treated with steroids, aged 1 to 10 years and 16 individuals treated with steroids, aged 7 to 10 years. We analyzed morphologic measures, normalized every measure for the average number of fibers observed for each year of age, and calculated intraclass correlation coefficients.
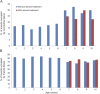
Results: The average proportion of connective tissue in patients not treated with steroids was 16.98% from ages 1 to 6 years and 30% from ages 7 to 10 years (p < 0.0001). The average proportion in patients treated with steroids was 24.90%. Muscle fiber area mirrored that of connective tissue in both groups.
Conclusions: Having provided a reproducible tool for evaluation and comparison of histologic changes occurring in patients undergoing clinical trials, it was observed that at ages 6 to 7 years, fibrotic tissue rapidly peaks to 29.85%; this is a crucial moment when muscle tissue loses its self-regeneration ability, veering toward fibrotic degeneration. These data should be considered when deciding the most suitable time to begin therapy.
© 2015 American Academy of Neurology.
Figures

References
-
- Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 2013;74:637–647. - PubMed
-
- ClinicalTrials.gov. Available at: www.Clinicaltrials.gov. Accessed February 20, 2015.
-
- Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med 2011;364:1513–1522. - PubMed
