Marine bacteria from the French Atlantic coast displaying high forming-biofilm abilities and different biofilm 3D architectures
- PMID: 26498445
- PMCID: PMC4619314
- DOI: 10.1186/s12866-015-0568-4
Marine bacteria from the French Atlantic coast displaying high forming-biofilm abilities and different biofilm 3D architectures
Abstract
Background: Few studies have reported the species composition of bacterial communities in marine biofilms formed on natural or on man-made existing structures. In particular, the roles and surface specificities of primary colonizers are largely unknown for most surface types. The aim of this study was to obtain potentially pioneering bacterial strains with high forming-biofilm abilities from two kinds of marine biofilms, collected from two different surfaces of the French Atlantic coast: an intertidal mudflat which plays a central role in aquaculture and a carbon steel structure of a harbour, where biofilms may cause important damages.
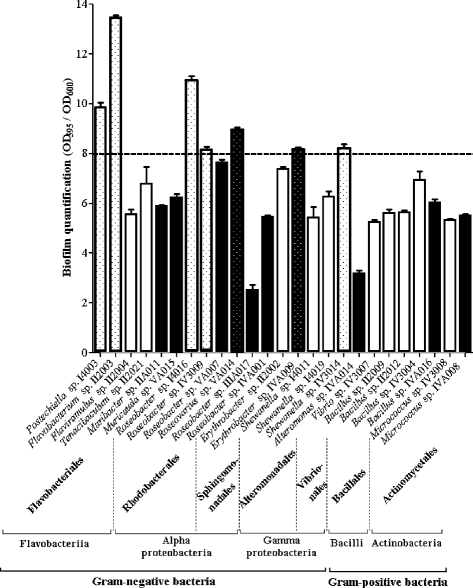
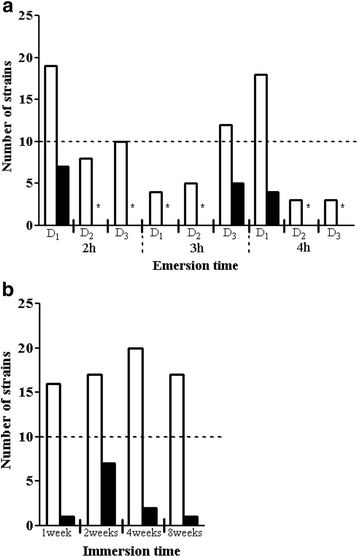
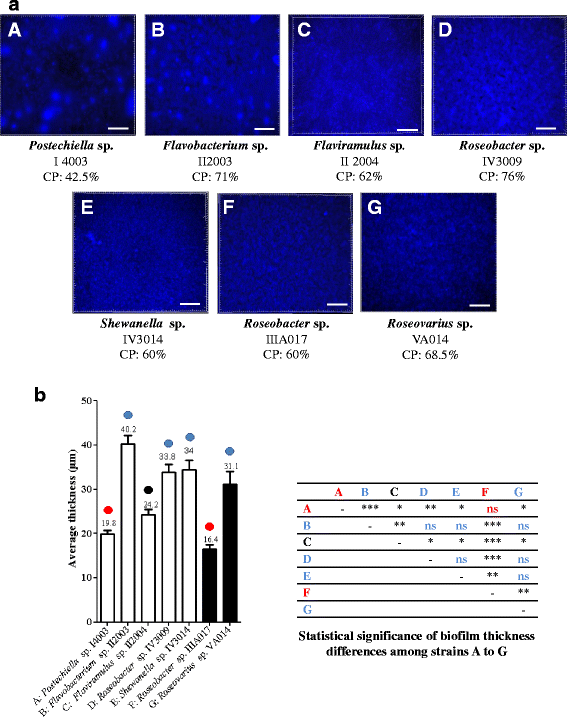
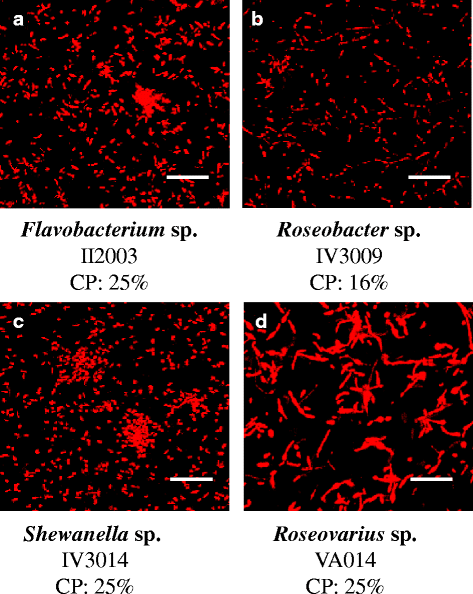
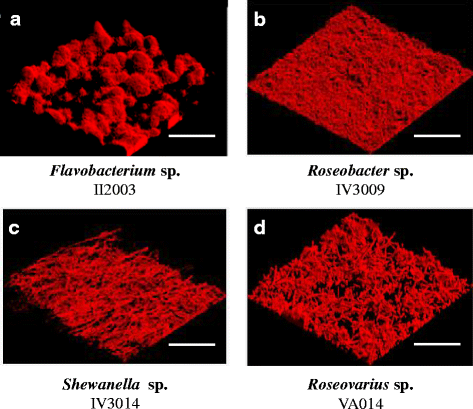
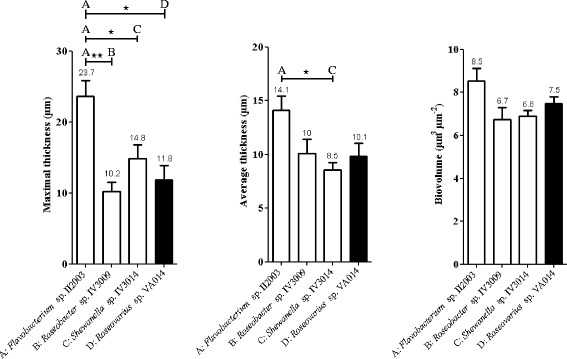
Results: A collection of 156 marine heterotrophic aerobic bacteria isolated from both biofilms was screened for their ability to form biofilms on polystyrene 96-well microtiter plates. Out of 25 strains able to build a biofilm in these conditions, only four bacteria also formed a thick and stable biofilm on glass surfaces under dynamic conditions. These strains developed biofilms with four different three-dimensional architectures when observed by confocal laser scanning microscopy: Flavobacterium sp. II2003 biofilms harboured mushroom-like structures, Roseobacter sp. IV3009 biofilms were quite homogeneous, Shewanella sp. IV3014 displayed hairy biofilms with horizontal fibres, whereas Roseovarius sp. VA014 developed heterogeneous and tousled biofilms.
Conclusions: This work led for the first time to the obtaining of four marine bacterial strains, potentially pioneering bacteria in marine biofilms, able to adhere to at least two different surfaces (polystyrene and glass) and to build specific 3D biofilms. The four selected strains are appropriate models for a better understanding of the colonization of a surface as well as the interactions that can occur between bacteria in a marine biofilm, which are crucial events for the initiation of biofouling.
Figures
Similar articles
-
Antibiofilm activity in the culture supernatant of a marine Pseudomonas sp. bacterium.Microbiology (Reading). 2020 Mar;166(3):239-252. doi: 10.1099/mic.0.000878. Microbiology (Reading). 2020. PMID: 31935186
-
Anti-Biofilm Activity of a Low Weight Proteinaceous Molecule from the Marine Bacterium Pseudoalteromonas sp. IIIA004 against Marine Bacteria and Human Pathogen Biofilms.Microorganisms. 2020 Aug 25;8(9):1295. doi: 10.3390/microorganisms8091295. Microorganisms. 2020. PMID: 32854286 Free PMC article.
-
Impacts of UV-C Irradiation on Marine Biofilm Community Succession.Appl Environ Microbiol. 2022 Feb 22;88(4):e0229821. doi: 10.1128/aem.02298-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936837 Free PMC article.
-
Ecological roles and biotechnological applications of marine and intertidal microbial biofilms.Adv Biochem Eng Biotechnol. 2014;146:163-205. doi: 10.1007/10_2014_271. Adv Biochem Eng Biotechnol. 2014. PMID: 24817086 Review.
-
Microbial Surface Colonization and Biofilm Development in Marine Environments.Microbiol Mol Biol Rev. 2015 Dec 23;80(1):91-138. doi: 10.1128/MMBR.00037-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2015. PMID: 26700108 Free PMC article. Review.
Cited by
-
A Novel Microbial Culture Chamber Co-cultivation System to Study Algal-Bacteria Interactions Using Emiliania huxleyi and Phaeobacter inhibens as Model Organisms.Front Microbiol. 2018 Jul 30;9:1705. doi: 10.3389/fmicb.2018.01705. eCollection 2018. Front Microbiol. 2018. PMID: 30105010 Free PMC article.
-
Bacterial community structure of early-stage biofilms is dictated by temporal succession rather than substrate types in the southern coastal seawater of India.PLoS One. 2021 Sep 27;16(9):e0257961. doi: 10.1371/journal.pone.0257961. eCollection 2021. PLoS One. 2021. PMID: 34570809 Free PMC article.
-
FlrA Represses Transcription of the Biofilm-Associated bpfA Operon in Shewanella putrefaciens.Appl Environ Microbiol. 2017 Feb 1;83(4):e02410-16. doi: 10.1128/AEM.02410-16. Print 2017 Feb 15. Appl Environ Microbiol. 2017. PMID: 27986717 Free PMC article.
-
Microbial community development on model particles in the deep sulfidic waters of the Black Sea.Environ Microbiol. 2021 Jun;23(6):2729-2746. doi: 10.1111/1462-2920.15024. Epub 2020 Apr 30. Environ Microbiol. 2021. PMID: 32291864 Free PMC article.
-
Distinct Temporal Succession of Bacterial Communities in Early Marine Biofilms in a Portuguese Atlantic Port.Front Microbiol. 2020 Aug 11;11:1938. doi: 10.3389/fmicb.2020.01938. eCollection 2020. Front Microbiol. 2020. PMID: 32849482 Free PMC article.
References
-
- Salta M, Wharton JA, Blache Y, Stokes KR, Briand JF. Marine biofilms on artificial surfaces: structure and dynamics. Environ Microbiol. 2013;15:2879–2893. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

