Expansion of quiescent lung adenocarcinoma CD8+ T cells by MUC1-8-mer peptide-T2 cell-β2 microglobulin complexes
- PMID: 26498650
- PMCID: PMC4699617
- DOI: 10.3892/or.2015.4328
Expansion of quiescent lung adenocarcinoma CD8+ T cells by MUC1-8-mer peptide-T2 cell-β2 microglobulin complexes
Abstract
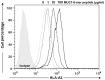
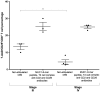
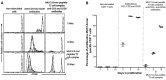
Adoptive immunotherapy requires the isolation of CD8+ T cells specific for tumor-associated antigens, their expansion in vitro and their transfusion to the patient to mediate a therapeutic effect. MUC1 is an important adenocarcinoma antigen immunogenic for T cells. The MUC1-derived SAPDTRPA (MUC1-8-mer) peptide is a potent epitope recognized by CD8+ T cells in murine models. Likewise, the T2 cell line has been used as an antigen-presenting cell to activate CD8+ T cells, but so far MUC1 has not been assessed in this context. We evaluated whether the MUC1-8-mer peptide can be presented by T2 cells to expand CD25+CD8+ T cells isolated from HLA-A2+ lung adenocarcinoma patients with stage III or IV tumors. The results showed that MUC1-8-mer peptide-loaded T2 cells activated CD8+ T cells from cancer HLA-A2+ patients when anti-CD2, anti-CD28 antibodies and IL-2 were added. The percentage of CD25+CD8+ T cells was 3-fold higher than those in the non-stimulated cells (P=0.018). HLA-A2+ patient cells showed a significant difference (2.3-fold higher) in activation status than HLA-A2+ healthy control cells (P=0.04). Moreover, 77.6% of MUC1-8-mer peptide-specific CD8+ T cells proliferated following a second stimulation with MUC1-8-mer peptide-loaded T2 cells after 10 days of cell culture. There were significant differences in the percentage of basal CD25+CD8+ T cells in relation to the cancer stage; this difference disappeared after MUC1-8-mer peptide stimulation. In conclusion, expansion of CD25+CD8+ T cells by MUC1-8 peptide-loaded T2 cells plus costimulatory signals via CD2, CD28 and IL-2 can be useful in adoptive immunotherapy.
Figures


Similar articles
-
The cytotoxic T cell response to peptide analogs of the HLA-A*0201-restricted MUC1 signal sequence epitope, M1.2.Cancer Immunol Immunother. 2007 Mar;56(3):287-301. doi: 10.1007/s00262-006-0191-1. Epub 2006 Jul 28. Cancer Immunol Immunother. 2007. PMID: 16874487 Free PMC article.
-
Novel breast-tumor-associated MUC1-derived peptides: characterization in Db-/- x beta2 microglobulin (beta2m) null mice transgenic for a chimeric HLA-A2.1/Db-beta2 microglobulin single chain.Int J Cancer. 2000 Feb 1;85(3):391-7. Int J Cancer. 2000. PMID: 10652432
-
Identification of three non-VNTR MUC1-derived HLA-A*0201-restricted T-cell epitopes that induce protective anti-tumor immunity in HLA-A2/K(b)-transgenic mice.Int J Cancer. 2001 Feb 1;91(3):385-92. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1051>3.0.co;2-z. Int J Cancer. 2001. PMID: 11169964
-
Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen.Immunol Rev. 2002 Oct;188:114-21. doi: 10.1034/j.1600-065x.2002.18810.x. Immunol Rev. 2002. PMID: 12445285 Review.
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
Cited by
-
Selection of a Single Domain Antibody, Specific for an HLA-Bound Epitope of the Mycobacterial Ag85B Antigen.Front Immunol. 2020 Oct 2;11:577815. doi: 10.3389/fimmu.2020.577815. eCollection 2020. Front Immunol. 2020. PMID: 33117380 Free PMC article.
References
-
- Nakamura H, Saji H. Worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today. 2014;44:1004–1012. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 8th edition. Elsevier Saunders; Philadelphia, PA: 2015. pp. 109–220.
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

