Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness
- PMID: 26498753
- PMCID: PMC4620013
- DOI: 10.1186/s12885-015-1780-1
Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness
Abstract
Background: Emerging evidence indicates that inappropriate cell-cell fusion might contribute to cancer progression. Similarly, mesenchymal stem cells (MSCs) can also fuse with other cells spontaneously and capable of adopting the phenotype of other cells. The aim of our study was to investigate the role of MSCs participated cell fusion in the tumorigenesis of gastric cancer.
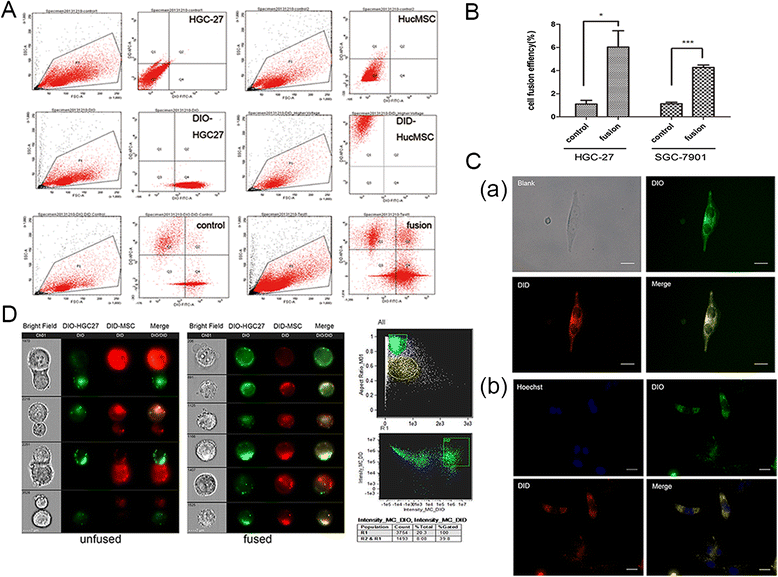
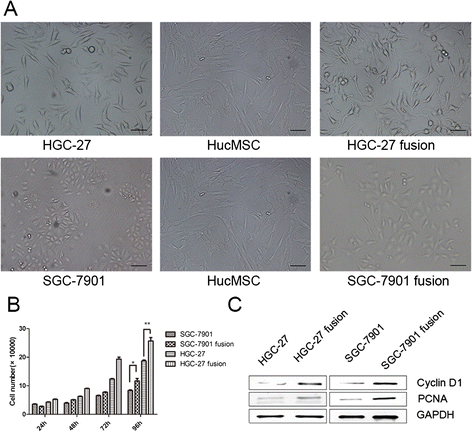
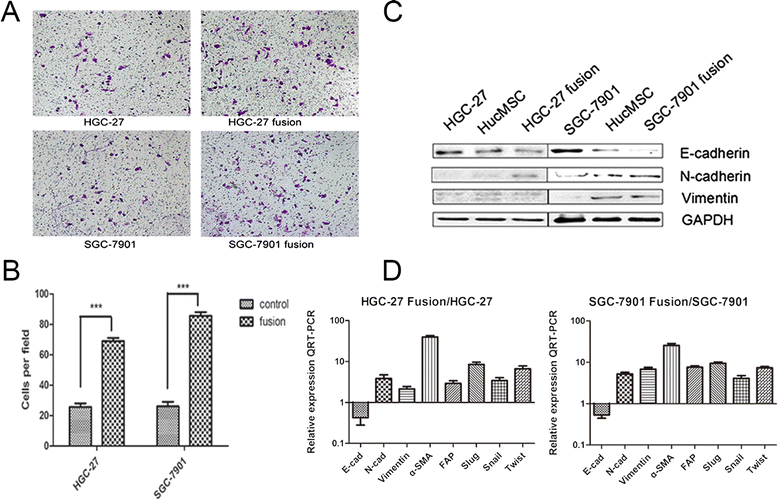
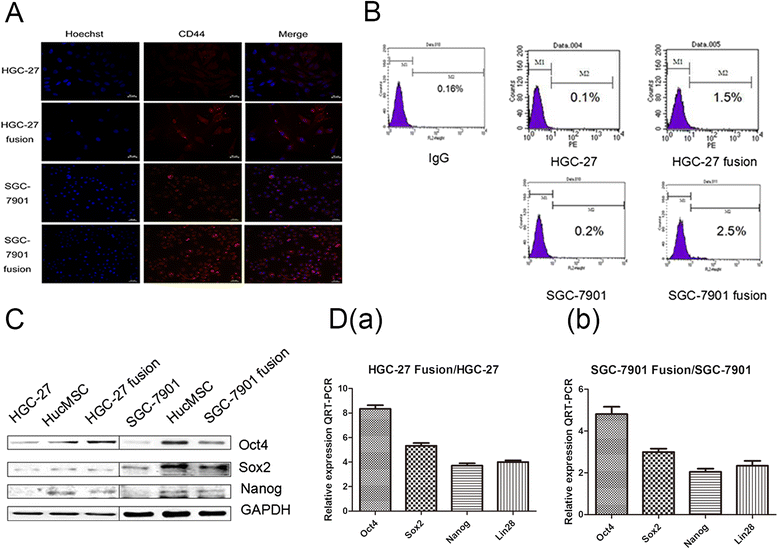
Methods: We fused human umbilical cord mesenchymal stem cells (hucMSCs) with gastric cancer cells in vitro by polyethylene glycol (PEG), the hybrid cells were sorted by flow cytometer. The growth and migration of hybrids were assessed by cell counting, cell colony formation and transwell assays. The proteins and genes related to epithelial- mesenchymal transition and stemness were tested by western blot, immunocytochemistry and real-time RT-PCR. The expression of CD44 and CD133 was examined by immunocytochemistry and flow cytometry. The xenograft assay was used to evaluation the tumorigenesis of the hybrids.
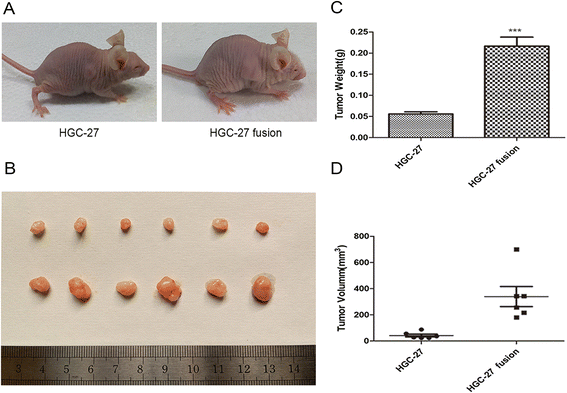
Results: The obtained hybrids exhibited epithelial- mesenchymal transition (EMT) change with down-regulation of E-cadherin and up-regulation of Vimentin, N-cadherin, α-smooth muscle actin (α-SMA), and fibroblast activation protein (FAP). The hybrids also increased expression of stemness factors Oct4, Nanog, Sox2 and Lin28. The expression of CD44 and CD133 on hybrid cells was stronger than parental gastric cancer cells. Moreover, the migration and proliferation of heterotypic hybrids were enhanced. In addition, the heterotypic hybrids promoted the growth abilities of gastric xenograft tumor in vivo.
Conclusions: Taken together, our results suggest that cell fusion between hucMSCs and gastric cancer cells could contribute to tumorigenic hybrids with EMT and stem cell-like properties, which may provide a flexible tool for investigating the roles of MSCs in gastric cancer.
Figures
Similar articles
-
Cell fusion between gastric epithelial cells and mesenchymal stem cells results in epithelial-to-mesenchymal transition and malignant transformation.BMC Cancer. 2015 Jan 30;15:24. doi: 10.1186/s12885-015-1027-1. BMC Cancer. 2015. PMID: 25633122 Free PMC article.
-
LGR5 Is a Gastric Cancer Stem Cell Marker Associated with Stemness and the EMT Signature Genes NANOG, NANOGP8, PRRX1, TWIST1, and BMI1.PLoS One. 2016 Dec 29;11(12):e0168904. doi: 10.1371/journal.pone.0168904. eCollection 2016. PLoS One. 2016. PMID: 28033430 Free PMC article.
-
Pre-treatment of human umbilical cord-derived mesenchymal stem cells with interleukin-6 abolishes their growth-promoting effect on gastric cancer cells.Int J Mol Med. 2015 Feb;35(2):367-75. doi: 10.3892/ijmm.2014.2019. Epub 2014 Dec 2. Int J Mol Med. 2015. PMID: 25483835 Free PMC article.
-
Stem cells in gastric cancer.World J Gastroenterol. 2015 Jan 7;21(1):112-23. doi: 10.3748/wjg.v21.i1.112. World J Gastroenterol. 2015. PMID: 25574084 Free PMC article. Review.
-
Role of epithelial-mesenchymal transition in gastric cancer initiation and progression.World J Gastroenterol. 2014 May 14;20(18):5403-10. doi: 10.3748/wjg.v20.i18.5403. World J Gastroenterol. 2014. PMID: 24833870 Free PMC article. Review.
Cited by
-
Efficacy of mesenchymal stem cells in the treatment of gastrointestinal malignancies.World J Gastrointest Oncol. 2020 Apr 15;12(4):365-382. doi: 10.4251/wjgo.v12.i4.365. World J Gastrointest Oncol. 2020. PMID: 32368316 Free PMC article. Review.
-
Targeting cancer stem cell pathways for cancer therapy.Signal Transduct Target Ther. 2020 Feb 7;5(1):8. doi: 10.1038/s41392-020-0110-5. Signal Transduct Target Ther. 2020. PMID: 32296030 Free PMC article. Review.
-
Novel Hybrid Phenotype Revealed in Small Cell Lung Cancer by a Transcription Factor Network Model That Can Explain Tumor Heterogeneity.Cancer Res. 2017 Mar 1;77(5):1063-1074. doi: 10.1158/0008-5472.CAN-16-1467. Epub 2016 Dec 8. Cancer Res. 2017. PMID: 27932399 Free PMC article.
-
Could gastrointestinal tumor-initiating cells originate from cell-cell fusion in vivo?World J Gastrointest Oncol. 2021 Feb 15;13(2):92-108. doi: 10.4251/wjgo.v13.i2.92. World J Gastrointest Oncol. 2021. PMID: 33643526 Free PMC article. Review.
-
Revisiting the role of mesenchymal stromal cells in cancer initiation, metastasis and immunosuppression.Exp Hematol Oncol. 2024 Jul 1;13(1):64. doi: 10.1186/s40164-024-00532-4. Exp Hematol Oncol. 2024. PMID: 38951845 Free PMC article. Review.
References
-
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80(3):267–74. doi:10.1016/j.yexmp.2005.07.004. - PubMed
-
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. doi:10.1016/j.canlet.2011.10.002. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

