Imaging Cytoskeleton Components by Electron Microscopy
- PMID: 26498781
- PMCID: PMC4841445
- DOI: 10.1007/978-1-4939-3124-8_5
Imaging Cytoskeleton Components by Electron Microscopy
Abstract
The cytoskeleton is a complex of detergent-insoluble components of the cytoplasm playing critical roles in cell motility, shape generation, and mechanical properties of a cell. Fibrillar polymers-actin filaments, microtubules, and intermediate filaments-are major constituents of the cytoskeleton, which constantly change their organization during cellular activities. The actin cytoskeleton is especially polymorphic, as actin filaments can form multiple higher order assemblies performing different functions. Structural information about cytoskeleton organization is critical for understanding its functions and mechanisms underlying various forms of cellular activity. Because of the nanometer-scale thickness of cytoskeletal fibers, electron microscopy (EM) is a key tool to determine the structure of the cytoskeleton. This article describes application of rotary shadowing (or metal replica) EM for visualization of the cytoskeleton. The procedure is applicable to thin cultured cells growing on glass coverslips and consists of detergent extraction of cells to expose their cytoskeleton, chemical fixation to provide stability, ethanol dehydration and critical point drying to preserve three-dimensionality, rotary shadowing with platinum to create contrast, and carbon coating to stabilize replicas. This technique provides easily interpretable three-dimensional images, in which individual cytoskeletal fibers are clearly resolved, and individual proteins can be identified by immunogold labeling. More importantly, replica EM is easily compatible with live cell imaging, so that one can correlate the dynamics of a cell or its components, e.g., expressed fluorescent proteins, with high resolution structural organization of the cytoskeleton in the same cell.
Keywords: Actin; Correlative microscopy; Critical point drying; Cytoskeleton; Electron microscopy; Immunogold; Microtubules; Rotary shadowing.
Figures

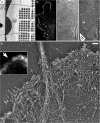
Republished from
- Methods Mol Biol.
References
-
- Wohlfarth-Bottermann KE. Weitreichende fibrillare Protoplasmadifferenzierungen und ihre Bedeutung fur die Protoplasmastromung. I. Elektronenmikroskopischer Nachweis und Feinstruktur. Protoplasma. 1962;54:514–539.
-
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67(2):359–367. - PubMed
-
- Wohlfarth-Bottermann KE. Differentiations of the ground cytoplasm and their significance for the generation of the motive force of amoeboid movement. In: Allen RD, Kamiya N, editors. Primitive motile systems in cell biology. Academic; New York: 1964. pp. 79–109.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

