Age modifies respiratory complex I and protein homeostasis in a muscle type-specific manner
- PMID: 26498839
- PMCID: PMC4717270
- DOI: 10.1111/acel.12412
Age modifies respiratory complex I and protein homeostasis in a muscle type-specific manner
Abstract
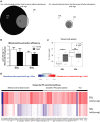
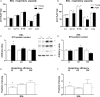
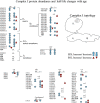
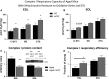
Changes in mitochondrial function with age vary between different muscle types, and mechanisms underlying this variation remain poorly defined. We examined whether the rate of mitochondrial protein turnover contributes to this variation. Using heavy label proteomics, we measured mitochondrial protein turnover and abundance in slow-twitch soleus (SOL) and fast-twitch extensor digitorum longus (EDL) from young and aged mice. We found that mitochondrial proteins were longer lived in EDL than SOL at both ages. Proteomic analyses revealed that age-induced changes in protein abundance differed between EDL and SOL with the largest change being increased mitochondrial respiratory protein content in EDL. To determine how altered mitochondrial proteomics affect function, we measured respiratory capacity in permeabilized SOL and EDL. The increased mitochondrial protein content in aged EDL resulted in reduced complex I respiratory efficiency in addition to increased complex I-derived H2 O2 production. In contrast, SOL maintained mitochondrial quality, but demonstrated reduced respiratory capacity with age. Thus, the decline in mitochondrial quality with age in EDL was associated with slower protein turnover throughout life that may contribute to the greater decline in mitochondrial dysfunction in this muscle. Furthermore, mitochondrial-targeted catalase protected respiratory function with age suggesting a causal role of oxidative stress. Our data clearly indicate divergent effects of age between different skeletal muscles on mitochondrial protein homeostasis and function with the greatest differences related to complex I. These results show the importance of tissue-specific changes in the interaction between dysregulation of respiratory protein expression, oxidative stress, and mitochondrial function with age.
Keywords: aging; mitochondria; mitochondrial dysfunction; protein turnover; proteome; skeletal muscle.
© 2015 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


Similar articles
-
Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles.Aging Cell. 2011 Dec;10(6):1047-55. doi: 10.1111/j.1474-9726.2011.00745.x. Aging Cell. 2011. PMID: 21933339
-
Reduced mitochondrial respiration and increased calcium deposits in the EDL muscle, but not in soleus, from 12-week-old dystrophic mdx mice.Sci Rep. 2019 Feb 13;9(1):1986. doi: 10.1038/s41598-019-38609-4. Sci Rep. 2019. PMID: 30760802 Free PMC article.
-
Proteomic analysis revealed different responses to hypergravity of soleus and extensor digitorum longus muscles in mice.J Proteomics. 2020 Apr 15;217:103686. doi: 10.1016/j.jprot.2020.103686. Epub 2020 Feb 12. J Proteomics. 2020. PMID: 32061808
-
Effect of cross-reinnervation on the expression of GLUT-4 and GLUT-1 in slow and fast rat muscles.Am J Physiol. 1996 Jun;270(6 Pt 2):R1355-60. doi: 10.1152/ajpregu.1996.270.6.R1355. Am J Physiol. 1996. PMID: 8764304
-
Age-related changes of aqueous protein profiles in rat fast and slow twitch skeletal muscles.Electrophoresis. 2000 Jan;21(2):465-72. doi: 10.1002/(SICI)1522-2683(20000101)21:2<465::AID-ELPS465>3.0.CO;2-5. Electrophoresis. 2000. PMID: 10675029
Cited by
-
Mitochondrial Quantity and Quality in Age-Related Sarcopenia.Int J Mol Sci. 2024 Feb 8;25(4):2052. doi: 10.3390/ijms25042052. Int J Mol Sci. 2024. PMID: 38396729 Free PMC article. Review.
-
Age-invariant genes: multi-tissue identification and characterization of murine reference genes.Aging (Albany NY). 2025 Jan 27;17(1):170-202. doi: 10.18632/aging.206192. Epub 2025 Jan 27. Aging (Albany NY). 2025. PMID: 39873648 Free PMC article.
-
Pan-PTM profiling identifies post-translational modifications associated with exceptional longevity and preservation of skeletal muscle function in Drosophila.NPJ Aging. 2025 Mar 30;11(1):23. doi: 10.1038/s41514-025-00215-2. NPJ Aging. 2025. PMID: 40159514 Free PMC article.
-
Accumulation of "Old Proteins" and the Critical Need for MS-based Protein Turnover Measurements in Aging and Longevity.Proteomics. 2020 Mar;20(5-6):e1800403. doi: 10.1002/pmic.201800403. Epub 2019 Sep 10. Proteomics. 2020. PMID: 31408259 Free PMC article. Review.
-
Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging.Aging (Albany NY). 2018 Nov 18;10(11):3327-3352. doi: 10.18632/aging.101643. Aging (Albany NY). 2018. PMID: 30449736 Free PMC article.
References
-
- Augusto V, Padovani CR, Rocha Campos GE (2004) Skeletal Muscle fiber Types in C57BL6J Mice. Braz. J. Morphol. Sci. 2, 89–94.
-
- Balsa E, Marco R, Perales‐Clemente E, Szklarczyk R, Calvo E, Landazuri M, Enriquez A (2012) NDUFA4 Is a Subunit of Complex IV of the Mammalian Electron Transport Chain. Cell Metabolism 16, 378–386. - PubMed
-
- Bortz WM 2nd (2002) A conceptual framework of frailty: a review. J. Gerontol. A Biol. Sci. Med. Sci. 57, M283–M288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

