Intermedin protects against renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress
- PMID: 26498843
- PMCID: PMC4619099
- DOI: 10.1186/s12882-015-0157-7
Intermedin protects against renal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress
Abstract
Background: Intermedin (IMD) is a novel member of the calcitonin/calcitonin gene-related peptide family. Endoplasmic reticulum stress (ERS) has been implicated in the pathology of renal ischemia/reperfusion (IRI). In the present study, we investigated whether IMD could reduce ERS damage after renal ischemia.
Methods: The kidneys of SD rats were subjected to 45 min of warm ischemia followed by 24 h of reperfusion. The hypoxia/reoxygenation(H/R) model in NRK-52E cells consisted of hypoxia for 1 h and reoxygenation for 2 h. IMD was over-expressed in vivo and in vitro using the vector pcDNA3.1-IMD. The serum creatinine concentration and lactate dehydrogenase (LDH) activity in the plasma were determined. Histologic examinations of renal tissues were performed with PAS staining. Real-time PCR and Western blotting were used to determine the mRNA and protein levels, respectively. Additionally, ER staining was used to detect the ERS response.
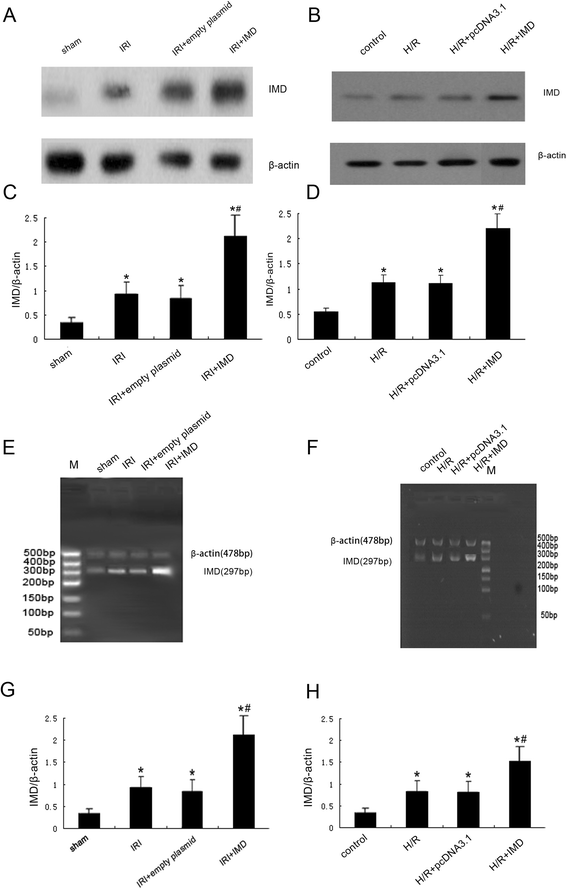
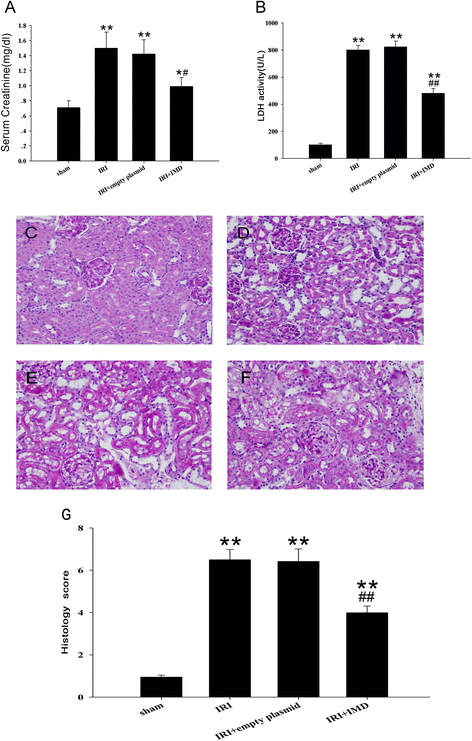
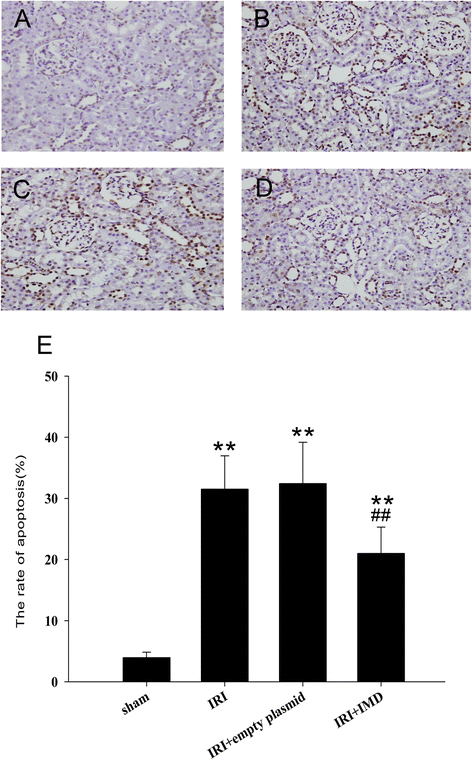
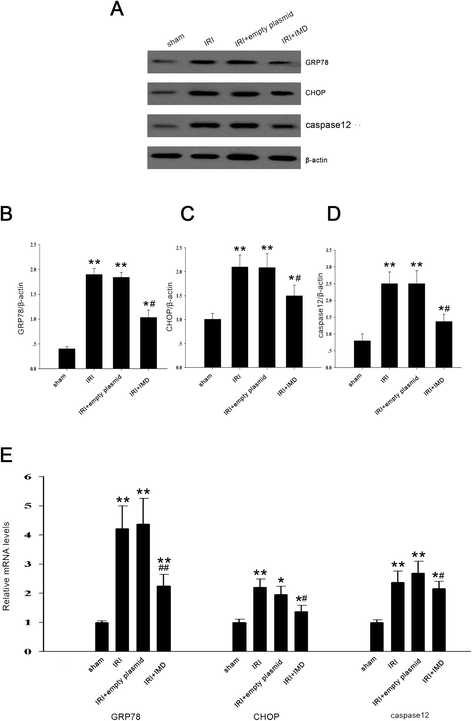
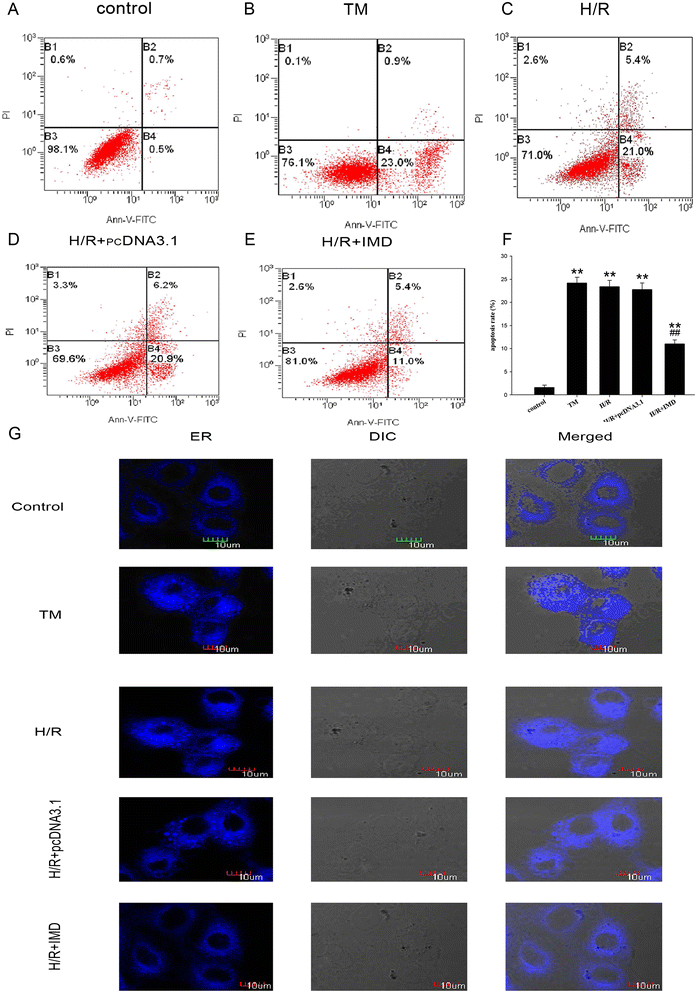
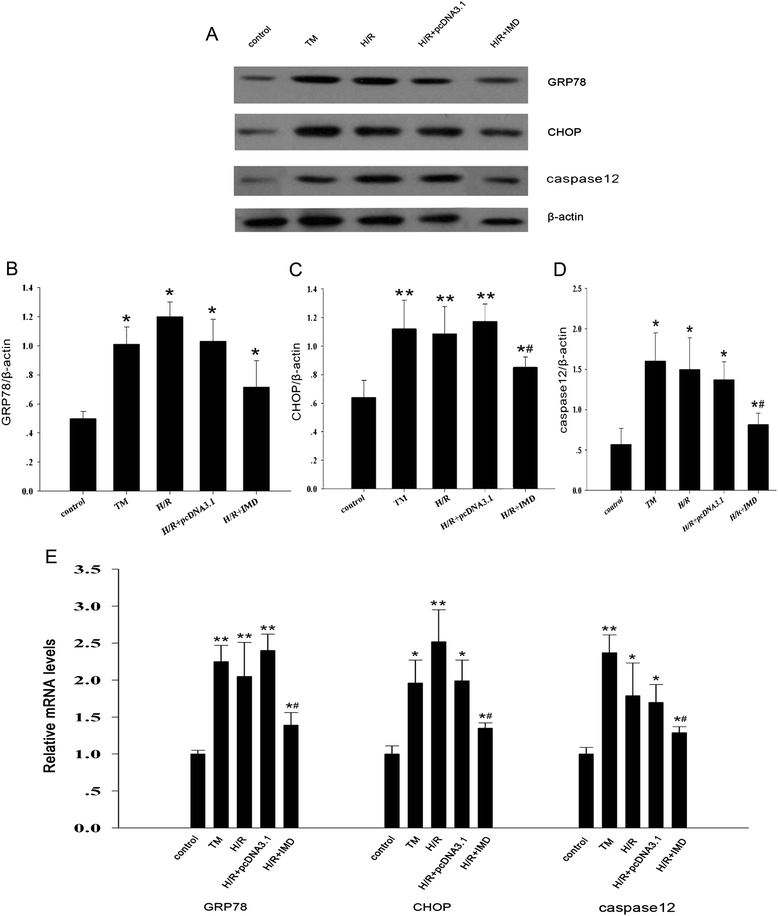
Results: In the rat renal IRI model, we found that IMD gene transfer markedly improved renal function and pathology and decreased LDH activity and cell apoptosis compared with the kidneys that were transfected with the control plasmid. IMD significantly attenuated the ERS stress parameters compared with IRI group. Indeed, IMD down-regulated glucose-regulated protein 78 (GRP78), C/EBP homologous protein(CHOP), and caspase 12 protein and mRNA levels. Moreover, in the NRK-52E cell H/R model, IMD overexpression prevented the apoptosis induced by H/R. Furthermore, IMD ameliorated the ER structural changes and concomitantly decreased the levels of GRP78, CHOP and caspase-12.
Conclusion: This study revealed that IMD protects against renal IRI by suppressing ERS and ERS-related apoptosis.
Figures
Similar articles
-
Intermedin/adrenomedullin 2 protects against tubular cell hypoxia-reoxygenation injury in vitro by promoting cell proliferation and upregulating cyclin D1 expression.Nephrology (Carlton). 2013 Sep;18(9):623-32. doi: 10.1111/nep.12114. Nephrology (Carlton). 2013. PMID: 23782291
-
Intermedin Alleviates Renal Ischemia-Reperfusion Injury and Enhances Neovascularization in Wistar Rats.Drug Des Devel Ther. 2020 Nov 10;14:4825-4834. doi: 10.2147/DDDT.S253019. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33204068 Free PMC article.
-
Inhibition of endoplasmic reticulum stress by intermedin(1-53) protects against myocardial injury through a PI3 kinase-Akt signaling pathway.J Mol Med (Berl). 2011 Dec;89(12):1195-205. doi: 10.1007/s00109-011-0808-5. Epub 2011 Sep 11. J Mol Med (Berl). 2011. PMID: 21909975
-
Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress.Am J Physiol Renal Physiol. 2013 Jan 1;304(1):F112-9. doi: 10.1152/ajprenal.00054.2012. Epub 2012 Oct 17. Am J Physiol Renal Physiol. 2013. PMID: 23077098
-
Multi-biological functions of intermedin in diseases.Front Physiol. 2023 Sep 6;14:1233073. doi: 10.3389/fphys.2023.1233073. eCollection 2023. Front Physiol. 2023. PMID: 37745233 Free PMC article. Review.
Cited by
-
MicroRNA-423-5p facilitates hypoxia/reoxygenation-induced apoptosis in renal proximal tubular epithelial cells by targeting GSTM1 via endoplasmic reticulum stress.Oncotarget. 2017 May 30;8(47):82064-82077. doi: 10.18632/oncotarget.18289. eCollection 2017 Oct 10. Oncotarget. 2017. PMID: 29137244 Free PMC article.
-
Connexin32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injury.J Transl Med. 2018 May 4;16(1):117. doi: 10.1186/s12967-018-1493-8. J Transl Med. 2018. PMID: 29728112 Free PMC article.
-
Edaravone Ameliorates Renal Warm Ischemia-Reperfusion Injury by Downregulating Endoplasmic Reticulum Stress in a Rat Resuscitation Model.Drug Des Devel Ther. 2020 Jan 15;14:175-183. doi: 10.2147/DDDT.S211906. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32021102 Free PMC article.
-
Intermedin attenuates renal fibrosis by induction of heme oxygenase-1 in rats with unilateral ureteral obstruction.BMC Nephrol. 2017 Jul 11;18(1):232. doi: 10.1186/s12882-017-0659-6. BMC Nephrol. 2017. PMID: 28697727 Free PMC article.
-
Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury.Ann Med. 2018 Aug;50(5):381-390. doi: 10.1080/07853890.2018.1489142. Epub 2018 Jul 11. Ann Med. 2018. PMID: 29895209 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

