Defining the complementarities between antibodies and haptens to refine our understanding and aid the prediction of a successful binding interaction
- PMID: 26498921
- PMCID: PMC4619568
- DOI: 10.1186/s12896-015-0217-x
Defining the complementarities between antibodies and haptens to refine our understanding and aid the prediction of a successful binding interaction
Abstract
Background: Low molecular weight haptens (<1000 Da) cannot be recognized by the immune system unless conjugated to larger carrier molecules. Antibodies to these exceptionally small antigens can still be generated with exquisite sensitivity. A detailed understanding at the molecular level of this incredible ability of antibodies to recognize haptens, is still limited compared to other antigen classes.
Methods: Different hapten targets with a broad range of structural flexibility and polarity were conjugated to carrier proteins, and utilized in sheep immunization. Three antibody libraries were constructed and used as potential pools to isolate specific antibodies to each target. The isolated antibodies were analysed in term of CDR length, canonical structure, and binding site shape and electrostatic potential.
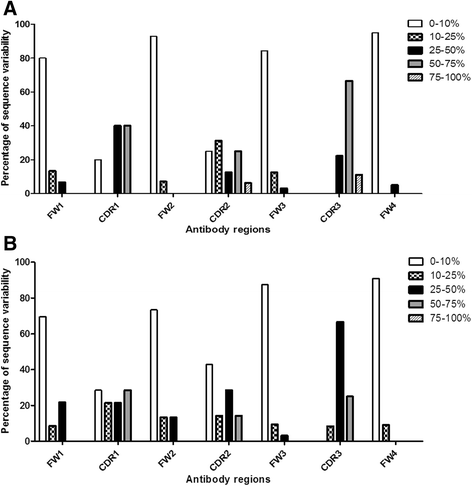
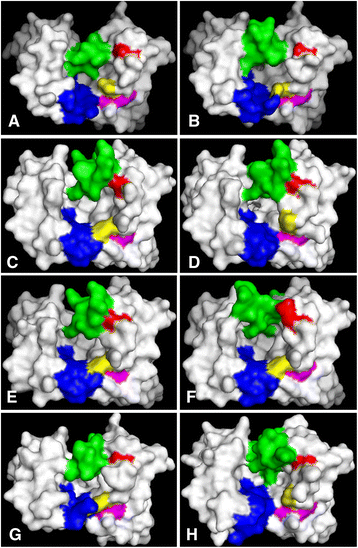
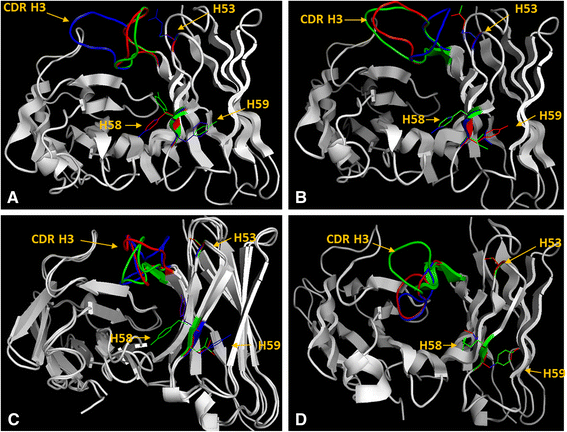
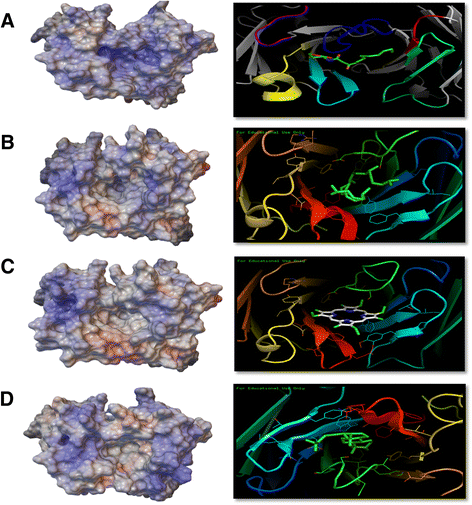
Results: The simple, chemically naïve structure of squalane (SQA) was recognized with micromolar sensitivity. An increase in structural rigidity of the hydrophobic and cyclic coprostane (COP) did not improve this binding sensitivity beyond the micromolar range, whilst the polar etioporphyrin (POR) was detected with nanomolar sensitivity. Homoserine lactone (HSL) molecules, which combine molecular flexibility and polarity, generated super-sensitive (picomolar) interactions. To better understand this range of antibody-hapten interactions, analyses were extended to examine the binding loop canonical structures and CDR lengths of a series of anti-hapten clones. Analyses of the pre and post- selection (panning of the phage displayed libraries) sequences revealed more conserved sites (123) within the post-selection sequences, when compared to their pre-selection counterparts (28). The strong selection pressure, generated by panning against these haptens resulted in the isolation of antibodies with significant sequence conservation in the FW regions, and suitable binding site cavities, representing only a relatively small subset of the available full repertoire sequence and structural diversity. As part of this process, the important influence of CDR H2 on antigen binding was observed through its direct interaction with individual antigens and indirect impact on the orientation and the pocket shape, when combined with CDRs H3 and L3. The binding pockets also displayed electrostatic surfaces that were complementary to the hydrophobic nature of COP, SQA, and POR, and the negatively charged HSL.
Conclusions: The best binding antibodies have shown improved capacity to recognize these haptens by establishing complementary binding pockets in terms of size, shape, and electrostatic potential.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

