The orphan nuclear receptor Nur77 inhibits low shear stress-induced carotid artery remodeling in mice
- PMID: 26498924
- PMCID: PMC4678158
- DOI: 10.3892/ijmm.2015.2375
The orphan nuclear receptor Nur77 inhibits low shear stress-induced carotid artery remodeling in mice
Abstract
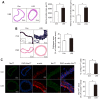
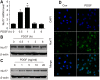
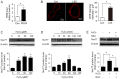
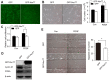
Shear stress, particularly low and oscillatory shear stress, plays a critical pathophysiological role in vascular remodeling-related cardiovascular diseases. Growing evidence suggests that the orphan nuclear receptor Nur77 [also known as TR3 or nuclear receptor subfamily 4, group A, member 1 (NR4A1)] is expressed in diseased human vascular tissue and plays an important role in vascular physiology and pathology. In the present study, we used a mouse model of flow-dependent remodeling by partial ligation of the left common carotid artery (LCCA) to define the exact role of Nur77 in vascular remodeling induced by low shear stress. Following vascular remodeling, Nur77 was highly expressed in neointimal vascular smooth muscle cells (VSMCs) in the ligated carotid arteries. The reactive oxygen species (ROS) levels were elevated in the remodeled arteries in vivo and in primary rat VSMCs in vitro following stimulation with platelet-derived growth factor (PDGF). Further in vitro experiments revealed that Nur77 expression was rapidly increased in the VSMCs following stimulation with PDGF and H2O2, whereas treatment with N-acetyl cysteine (NAC, a ROS scavenger) reversed the increase in the protein level of Nur77 induced by H2O2. Moreover, Nur77 overexpression markedly inhibited the proliferation and migration of VSMCs, induced by PDGF. Finally, to determine the in vivo role of Nur77 in low shear stress-induced vascular remodeling, wild-type (WT) and Nur77-deficient mice were subjected to partial ligation of the LCCA. Four weeks following surgery, in the LCCAs of the Nur77‑deficient mice, a significant increase in the intima-media area and carotid intima-media thickness was noted, as well as more severe elastin disruption and collagen deposition compared to the WT mice. Immunofluorescence staining revealed an increase in VSMC proliferation [determined by the expression of proliferating cell nuclear antigen (PCNA)] and matrix metalloproteinase 9 (MMP-9) production in the Nur77-deficient mice. There was no difference in the number of intimal apoptotic cells between the groups. Taken together, our results indicate that Nur77 may be a sensor of oxidative stress and an inhibitor of vascular remodeling induced by low shear stress. Nur77, as well as its downstream cell signals, may thus be a potential therapeutic target for the suppression of vascular remodeling.
Figures


Similar articles
-
Orphan Nuclear Receptor Nur77 Inhibits Angiotensin II-Induced Vascular Remodeling via Downregulation of β-Catenin.Hypertension. 2016 Jan;67(1):153-62. doi: 10.1161/HYPERTENSIONAHA.115.06114. Epub 2015 Nov 23. Hypertension. 2016. PMID: 26597820
-
MFAP4 Promotes Vascular Smooth Muscle Migration, Proliferation and Accelerates Neointima Formation.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):122-33. doi: 10.1161/ATVBAHA.115.306672. Epub 2015 Nov 12. Arterioscler Thromb Vasc Biol. 2016. PMID: 26564819
-
Nur77 downregulation triggers pulmonary artery smooth muscle cell proliferation and migration in mice with hypoxic pulmonary hypertension via the Axin2-β-catenin signaling pathway.Vascul Pharmacol. 2016 Dec;87:230-241. doi: 10.1016/j.vph.2016.11.002. Epub 2016 Nov 15. Vascul Pharmacol. 2016. PMID: 27871853
-
Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis.Int J Mol Sci. 2021 Sep 30;22(19):10585. doi: 10.3390/ijms221910585. Int J Mol Sci. 2021. PMID: 34638923 Free PMC article. Review.
-
Characteristics of Nur77 and its ligands as potential anticancer compounds (Review).Mol Med Rep. 2018 Dec;18(6):4793-4801. doi: 10.3892/mmr.2018.9515. Epub 2018 Sep 27. Mol Med Rep. 2018. PMID: 30272297 Free PMC article. Review.
Cited by
-
SENP2 Promotes VSMC Phenotypic Switching via Myocardin De-SUMOylation.Int J Mol Sci. 2022 Oct 20;23(20):12637. doi: 10.3390/ijms232012637. Int J Mol Sci. 2022. PMID: 36293488 Free PMC article.
-
Function of Nr4a Orphan Nuclear Receptors in Proliferation, Apoptosis and Fuel Utilization Across Tissues.Cells. 2019 Nov 1;8(11):1373. doi: 10.3390/cells8111373. Cells. 2019. PMID: 31683815 Free PMC article. Review.
-
5-Aminosalicylic Acid Attenuates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats by Increasing the Expression of Nur77.Inflammation. 2017 Jun;40(3):806-817. doi: 10.1007/s10753-017-0525-5. Inflammation. 2017. PMID: 28213866
-
Nuclear receptor subfamily 4 group a member 1 eases angiotensin II-arose oxidative stress in vascular smooth muscle cell by boosting nucleotide-binding oligomerization domain-like receptor family caspase recruitment domain containing 3 transcription.Cytojournal. 2024 Nov 16;21:43. doi: 10.25259/Cytojournal_86_2024. eCollection 2024. Cytojournal. 2024. PMID: 39737121 Free PMC article.
-
NR4A1 deficiency promotes carotid plaque vulnerability by activating integrated stress response via targeting Bcat1.Cell Mol Life Sci. 2025 Feb 22;82(1):91. doi: 10.1007/s00018-025-05602-2. Cell Mol Life Sci. 2025. PMID: 39985585 Free PMC article.
References
-
- Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity RG, Daley W, Rogers C, Edelman ER, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. - DOI - PubMed
-
- Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. A study in the normal rabbit and the hypercholesterolemic Yucatan micropig. Circulation. 1994;89:2816–2821. doi: 10.1161/01.CIR.89.6.2816. - DOI - PubMed
-
- Qi YX, Jiang J, Jiang XH, Wang XD, Ji SY, Han Y, Long DK, Shen BR, Yan ZQ, Chien S, et al. PDGF-BB and TGF-β1 on cross-talk between endothelial and smooth muscle cells in vascular remodeling induced by low shear stress. Proc Natl Acad Sci USA. 2011;108:1908–1913. doi: 10.1073/pnas.1019219108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

