Antioxidant Rescue of Selenomethionine-Induced Teratogenesis in Zebrafish Embryos
- PMID: 26498942
- PMCID: PMC4842345
- DOI: 10.1007/s00244-015-0235-7
Antioxidant Rescue of Selenomethionine-Induced Teratogenesis in Zebrafish Embryos
Erratum in
-
Erratum to: Antioxidant Rescue of Selenomethionine-Induced Teratogenesis in Zebrafish Embryos.Arch Environ Contam Toxicol. 2016 Oct;71(3):437. doi: 10.1007/s00244-016-0306-4. Arch Environ Contam Toxicol. 2016. PMID: 27491869 No abstract available.
Abstract
Selenium (Se) is an essential micronutrient that can be found at toxic concentrations in surface waters contaminated by runoff from agriculture and coal mining. Zebrafish (Danio rerio) embryos were exposed to aqueous Se in the form of selenate, selenite, and l-selenomethionine (SeMet) in an attempt to determine if oxidative stress plays a role in selenium embryo toxicity. Selenate and selenite exposure did not induce embryo deformities (lordosis and craniofacial malformation). l-selenomethionine, however, induced significantly higher deformity rates at 100 µg/L compared with controls. SeMet exposure induced a dose-dependent increase in the catalytic subunit of glutamate-cysteine ligase (gclc) and reached an 11.7-fold increase at 100 µg/L. SeMet exposure also reduced concentrations of TGSH, RGSH, and the TGSH:GSSG ratio. Pretreatment with 100 µM N-acetylcysteine significantly reduced deformities in the zebrafish embryos secondarily treated with 400 µg/L SeMet from approximately 50–10 % as well as rescued all three of the significant glutathione level differences seen with SeMet alone. Selenite exposure induced a 6.6-fold increase in expression of the glutathione-S-transferase pi class 2 (gstp2) gene, which is involved in xenobiotic transformation and possibly oxidative stress. These results suggest that aqueous exposure to SeMet can induce significant embryonic teratogenesis in zebrafish that are at least partially attributed to oxidative stress.
Figures
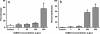


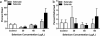

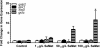
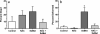
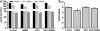
References
-
- Bergeron CM, Bodinof CM, Unrine JM, Hopkins WA. Bioaccumulation and maternal transfer of mercury and selenium in amphibians. Environ Toxicol Chem. 2010;29:989–997. doi:Doi 10.1002/Etc.125. - PubMed
-
- Bierl C, Voetsch B, Jin RC, Handy DE, Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi:DOI 10.1074/jbc.M401907200. - PubMed
-
- Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O. The role of glutathione status in the protection against ischemic and reperfusion damage - effects of N-acetyl cysteine. J Molec and Cell Cardiol. 1988;20:5–13. doi:Doi 10.1016/S0022-2828(88)80174-3. - PubMed
-
- Deflora S, Bennicelli C, Camoirano A, et al. In vivo effects of N-acetylcysteine on glutathione metabolism and on the biotransformation of carcinogenic and or mutagenic compounds. Carcinogenesis. 1985;6:1735–1745. - PubMed
-
- Di Giulio RT, Meyer JN. Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC Press; Boca Raton: 2008. pp. 273–324.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

