Time-multiplexed two-channel capacitive radiofrequency hyperthermia with nanoparticle mediation
- PMID: 26499058
- PMCID: PMC4619487
- DOI: 10.1186/s12938-015-0090-9
Time-multiplexed two-channel capacitive radiofrequency hyperthermia with nanoparticle mediation
Abstract
Background: Capacitive radiofrequency (RF) hyperthermia suffers from excessive temperature rise near the electrodes and poorly localized heat transfer to the deep-seated tumor region even though it is known to have potential to cure ill-conditioned tumors. To better localize heat transfer to the deep-seated target region in which electrical conductivity is elevated by nanoparticle mediation, two-channel capacitive RF heating has been tried on a phantom.
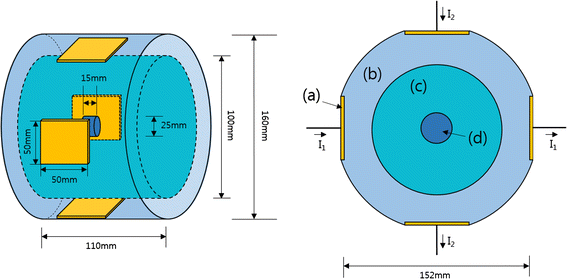
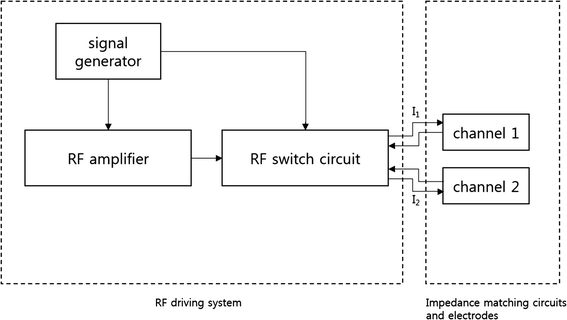
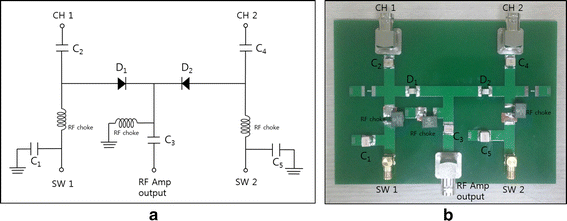
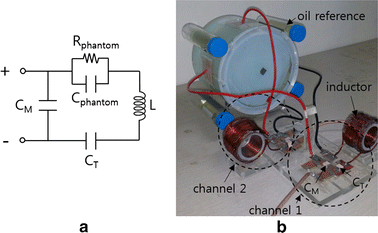
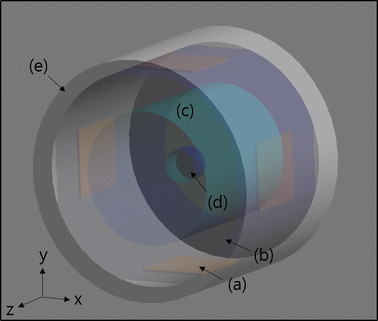
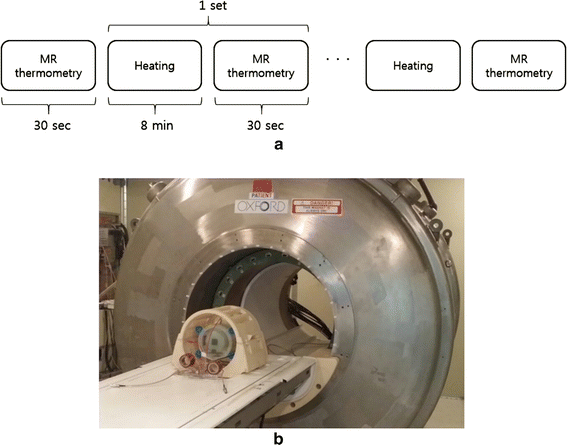
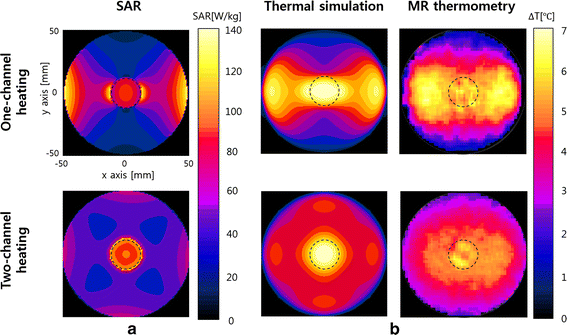
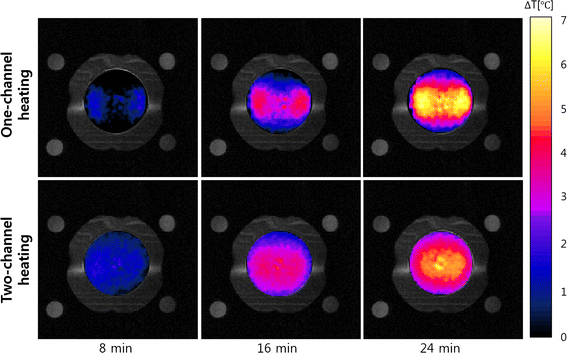
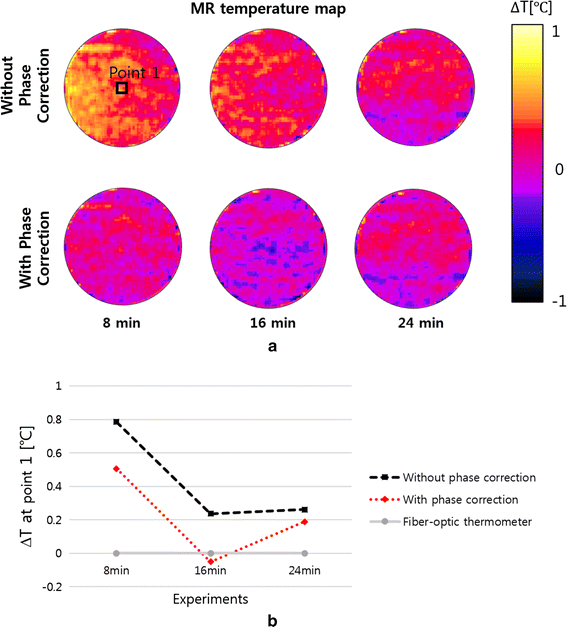
Methods: We made a tissue-mimicking phantom consisting of two compartments, a tumor-tissue-mimicking insert against uniform background agarose. The tumor-tissue-mimicking insert was made to have higher electrical conductivity than the normal-tissue-mimicking background by applying magnetic nanoparticle suspension to the insert. Two electrode pairs were attached on the phantom surface by equal-angle separation to apply RF electric field to the phantom. To better localize heat transfer to the tumor-tissue-mimicking insert, RF power with a frequency of 26 MHz was delivered to the two channels in a time-multiplexed way. To monitor the temperature rise inside the phantom, MR thermometry was performed at a 3T MRI intermittently during the RF heating. Finite-difference-time-domain (FDTD) electromagnetic and thermal simulations on the phantom model were also performed to verify the experimental results.
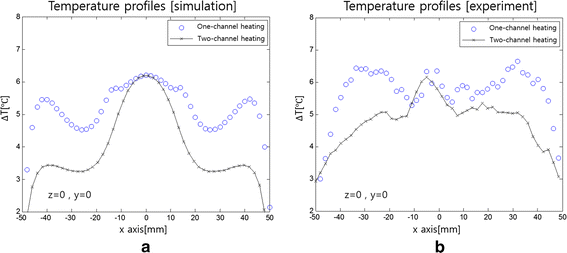
Results: As compared to the one-channel RF heating, the two-channel RF heating with time-multiplexed driving improved the spatial localization of heat transfer to the tumor-tissue-mimicking region in both the simulation and experiment. The two-channel RF heating also reduced the temperature rise near the electrodes significantly.
Conclusions: Time-multiplexed two-channel capacitive RF heating has the capability to better localize heat transfer to the nanoparticle-mediated tumor region which has higher electrical conductivity than the background normal tissues.
Figures


References
-
- Baronzio G, Parmar G, Ballerini M, Szasz A, Baronzio M, Cassutti V. A brief overview of hyperthermia in cancer treatment. J Integr Oncol. 2014;3:1–10. doi: 10.4172/2329-6771.1000115. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

