Neuronal response in Alzheimer's and Parkinson's disease: the effect of toxic proteins on intracellular pathways
- PMID: 26499115
- PMCID: PMC4619058
- DOI: 10.1186/s12868-015-0211-1
Neuronal response in Alzheimer's and Parkinson's disease: the effect of toxic proteins on intracellular pathways
Abstract
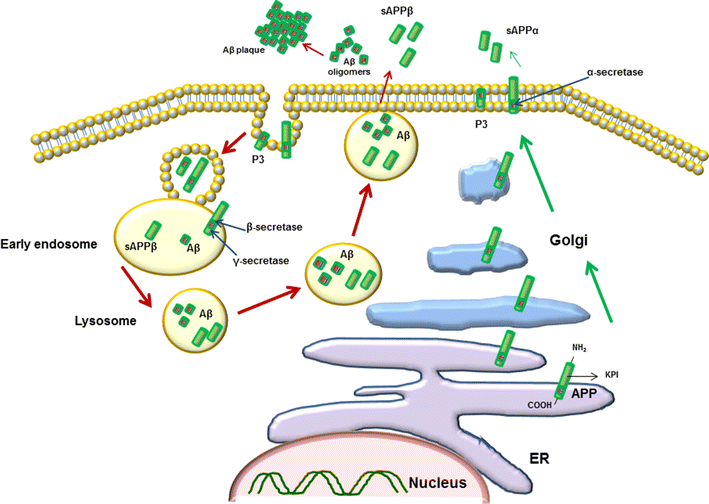
Accumulation of protein aggregates is the leading cause of cellular dysfunction in neurodegenerative disorders. Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease, Prion disease and motor disorders such as amyotrophic lateral sclerosis, present with a similar pattern of progressive neuronal death, nervous system deterioration and cognitive impairment. The common characteristic is an unusual misfolding of proteins which is believed to cause protein deposition and trigger degenerative signals in the neurons. A similar clinical presentation seen in many neurodegenerative disorders suggests the possibility of shared neuronal responses in different disorders. Despite the difference in core elements of deposits in each neurodegenerative disorder, the cascade of neuronal reactions such as activation of glycogen synthase kinase-3 beta, mitogen-activated protein kinases, cell cycle re-entry and oxidative stress leading to a progressive neurodegeneration are surprisingly similar. This review focuses on protein toxicity in two neurodegenerative diseases, AD and PD. We reviewed the activated mechanisms of neurotoxicity in response to misfolded beta-amyloid and α-synuclein, two major toxic proteins in AD and PD, leading to neuronal apoptosis. The interaction between the proteins in producing an overlapping pathological pattern will be also discussed.
Figures



References
-
- Karpinar DP, Balija MB, Kugler S, Opazo F, Rezaei-Ghaleh N, Wender N, Kim HY, Taschenberger G, Falkenburger BH, Heise H, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28(20):3256–3268. doi: 10.1038/emboj.2009.257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

