Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells
- PMID: 26499488
- PMCID: PMC4678157
- DOI: 10.3892/ijmm.2015.2378
Puquitinib mesylate (XC-302) induces autophagy via inhibiting the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells
Abstract
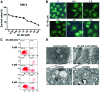
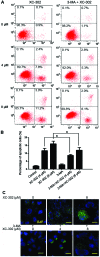
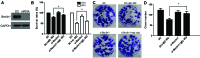
There are numerous studies that demonstrate the anti-neoplastic activity of phosphatidylinositol 3-kinase (PI3K) inhibitors and the mechanisms of inducing autophagy in cancer cells. The new anticancer drug puquitinib mesylate (XC-302) is a molecular-targeted drug, which suppresses the activity of PI3K directly. However, it remains unclear whether XC‑302 can develop an antitumor effect by inducing autophagy in nasopharyngeal cancer cells. The MTT assay was used to study the anti-proliferative effects of XC-302. Subsequently, autophagy was determined by monodansylcadaverine (MDC) staining, punctate localization of green fluorescent protein (GFP)-light chain 3 (LC3), LC3 protein blotting and electron microscopy. The expression levels of beclin 1, p62, protein kinase B (AKT), phospho (p)‑AKT, mechanistic target of rapamycin (mTOR) and p‑mTOR in XC-302‑induced autophagy were detected. Autophagy inhibition was assayed by 3-methyladenine (3‑MA) or small interfering RNA (siRNA) silencing of beclin 1. XC-302 inhibited the viability of CNE‑2 in a dose-dependent manner and the IC50 of 72 h was 5.2 µmol/l. After cells were exposed to XC-302 for 24 h, MDC-labeled autophagolysosomes were evident in CNE-2 cells by fluorescence microscope. Autophagosomes and autolysosomes were identified by transmission electron microscopy. Following transfection with GFP‑LC3, XC-302 induced a significant accumulation of GFP‑LC3, as monitored by a confocal microscope, which was reduced by 3-MA. XC-302 induced the formation of LC3‑II, increased beclin 1 levels and decreased the expression of p62. Additionally, the expression levels of p‑AKT and p‑mTOR were reduced with the elevation of XC-302. Knockdown of beclin 1 with siRNA or co-treatment with 3-MA enhanced significantly the survival of CNE-2 and promoted the ability of clone formation. XC-302 also induced apoptosis in CNE-2, and when autophagy was inhibited by 3-MA, the apoptosis rate was decreased. The present data provides the evidence that XC-302 can induce autophagy in CNE-2, which promotes the program of cell death and inhibits the PI3K/AKT/mTOR signaling pathway. Furthermore, XC-302 also promoted apoptosis in CNE-2 cells, which could be reduced when autophagy was suppressed, meaning that autophagy may interact with apoptosis to induce cell death.
Figures



Similar articles
-
Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation.World J Gastroenterol. 2013 Mar 21;19(11):1736-48. doi: 10.3748/wjg.v19.i11.1736. World J Gastroenterol. 2013. PMID: 23555162 Free PMC article.
-
PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase.Clin Cancer Res. 2011 Aug 15;17(16):5353-66. doi: 10.1158/1078-0432.CCR-10-1948. Epub 2011 Jun 20. Clin Cancer Res. 2011. Retraction in: Clin Cancer Res. 2013 Sep 1;19(17):4900. doi: 10.1158/1078-0432.CCR-13-1910. PMID: 21690574 Retracted.
-
Effects of small interfering RNA inhibit Class I phosphoinositide 3-kinase on human gastric cancer cells.World J Gastroenterol. 2013 Mar 21;19(11):1760-9. doi: 10.3748/wjg.v19.i11.1760. World J Gastroenterol. 2013. PMID: 23555164 Free PMC article.
-
Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway.Pharmacol Res Perspect. 2023 Feb;11(1):e01041. doi: 10.1002/prp2.1041. Pharmacol Res Perspect. 2023. PMID: 36572650 Free PMC article. Review.
-
Effects of metformin on cancers in experimental and clinical studies: Focusing on autophagy and AMPK/mTOR signaling pathways.Cell Biochem Funct. 2024 Jun;42(4):e4071. doi: 10.1002/cbf.4071. Cell Biochem Funct. 2024. PMID: 38863255 Review.
Cited by
-
Autophagy-Related Beclin 1 and Head and Neck Cancers.Onco Targets Ther. 2020 Jun 30;13:6213-6227. doi: 10.2147/OTT.S256072. eCollection 2020. Onco Targets Ther. 2020. PMID: 32669852 Free PMC article. Review.
-
Pharmacological Effects of Grifolin: Focusing on Anticancer Mechanisms.Molecules. 2022 Jan 3;27(1):284. doi: 10.3390/molecules27010284. Molecules. 2022. PMID: 35011516 Free PMC article. Review.
-
Lithium prevents glucocorticoid-induced osteonecrosis of the femoral head by regulating autophagy.J Cell Mol Med. 2024 May;28(10):e18385. doi: 10.1111/jcmm.18385. J Cell Mol Med. 2024. PMID: 38801405 Free PMC article.
-
RLIP76 Depletion Enhances Autophagic Flux in U251 Cells.Cell Mol Neurobiol. 2017 Apr;37(3):555-562. doi: 10.1007/s10571-016-0410-z. Epub 2016 Jul 29. Cell Mol Neurobiol. 2017. PMID: 27473470 Free PMC article.
-
Study on the multitarget mechanism of alliin activating autophagy based on network pharmacology and molecular docking.J Cell Mol Med. 2022 Nov;26(22):5590-5601. doi: 10.1111/jcmm.17573. Epub 2022 Oct 21. J Cell Mol Med. 2022. PMID: 36271672 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

