Astroglial β-Arrestin1-mediated Nuclear Signaling Regulates the Expansion of Neural Precursor Cells in Adult Hippocampus
- PMID: 26500013
- PMCID: PMC4620451
- DOI: 10.1038/srep15506
Astroglial β-Arrestin1-mediated Nuclear Signaling Regulates the Expansion of Neural Precursor Cells in Adult Hippocampus
Abstract
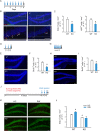
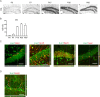
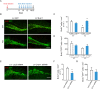
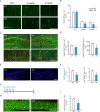
Adult hippocampal neurogenesis is crucial for preserving normal brain function, but how it is regulated by niche cells is uncertain. Here we show that β-arrestin 1 (β-arr1) in dentate gyrus (DG) regulates neural precursor proliferation. β-arr1 knockout (KO) mice show reduced neural precursor proliferation in subgranular zone (SGZ) which could be rescued by selective viral expression of β-arr1 but not its nuclear-function-deficient mutants under control of hGFAP promotor in DG. Compared with wild type astrocytes, β-arr1 KO astrocytes nurture less neurospheres, and this may be attributed to changed activity of soluble, heat-sensitive excretive factors, such as BMP2. RNA-sequencing reveals that β-arr1 KO DG astrocytes exhibit an aberrant gene expression profile of niche factors, including elevated transcription of Bmp2. Taken together, our data suggest that β-arr1 mediated nuclear signaling regulates the production of excretive factors derived from niche astrocytes and expansion of neural precursors in DG, thus maintaining homeostasis of adult hippocampal neurogenesis.
Figures

Similar articles
-
beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking.Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1601-6. doi: 10.1073/pnas.98.4.1601. Epub 2001 Feb 6. Proc Natl Acad Sci U S A. 2001. PMID: 11171997 Free PMC article.
-
Non-Hematopoietic β-Arrestin1 Confers Protection Against Experimental Colitis.J Cell Physiol. 2016 May;231(5):992-1000. doi: 10.1002/jcp.25216. Epub 2015 Nov 20. J Cell Physiol. 2016. PMID: 26479868 Free PMC article.
-
β-arrestin-1 is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling.Oncogene. 2013 Oct 17;32(42):5066-77. doi: 10.1038/onc.2012.527. Epub 2012 Dec 3. Oncogene. 2013. PMID: 23208497
-
Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus.Redox Biol. 2017 Oct;13:393-401. doi: 10.1016/j.redox.2017.06.010. Epub 2017 Jun 27. Redox Biol. 2017. PMID: 28667908 Free PMC article.
-
Rapid xenograft tumor progression in beta-arrestin1 transgenic mice due to enhanced tumor angiogenesis.FASEB J. 2008 Feb;22(2):355-64. doi: 10.1096/fj.07-9046com. Epub 2007 Sep 21. FASEB J. 2008. PMID: 17890288
Cited by
-
Double life: How GRK2 and β-arrestin signaling participate in diseases.Cell Signal. 2022 Jun;94:110333. doi: 10.1016/j.cellsig.2022.110333. Epub 2022 Apr 14. Cell Signal. 2022. PMID: 35430346 Free PMC article. Review.
-
Survey of transcriptome analyses of hippocampal neurogenesis with focus on adult dentate gyrus stem cells.Front Cell Dev Biol. 2025 May 30;13:1605116. doi: 10.3389/fcell.2025.1605116. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519263 Free PMC article. Review.
-
β-arrestin 2 is essential for fluoxetine-mediated promotion of hippocampal neurogenesis in a mouse model of depression.Acta Pharmacol Sin. 2021 May;42(5):679-690. doi: 10.1038/s41401-020-00576-2. Epub 2021 Feb 1. Acta Pharmacol Sin. 2021. PMID: 33526871 Free PMC article.
-
Current Understanding of the Neural Stem Cell Niches.Cells. 2022 Sep 26;11(19):3002. doi: 10.3390/cells11193002. Cells. 2022. PMID: 36230964 Free PMC article. Review.
-
Key Metabolic Functions of β-Arrestins: Studies with Novel Mouse Models.Trends Endocrinol Metab. 2021 Feb;32(2):118-129. doi: 10.1016/j.tem.2020.11.008. Epub 2020 Dec 23. Trends Endocrinol Metab. 2021. PMID: 33358450 Free PMC article. Review.
References
-
- Zhao C., Deng W. & Gage F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

