Astrocyte matricellular proteins that control excitatory synaptogenesis are regulated by inflammatory cytokines and correlate with paralysis severity during experimental autoimmune encephalomyelitis
- PMID: 26500475
- PMCID: PMC4598482
- DOI: 10.3389/fnins.2015.00344
Astrocyte matricellular proteins that control excitatory synaptogenesis are regulated by inflammatory cytokines and correlate with paralysis severity during experimental autoimmune encephalomyelitis
Abstract
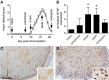
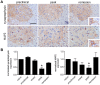
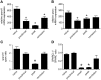
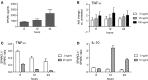
The matricellular proteins, secreted protein acidic and rich in cysteine (SPARC) and SPARC-like 1 (SPARCL1), are produced by astrocytes and control excitatory synaptogenesis in the central nervous system. While SPARCL1 directly promotes excitatory synapse formation in vitro and in the developing nervous system in vivo, SPARC specifically antagonizes the synaptogenic actions of SPARCL1. We hypothesized these proteins also help maintain existing excitatory synapses in adult hosts, and that local inflammation in the spinal cord alters their production in a way that dynamically modulates motor synapses and impacts the severity of paralysis during experimental autoimmune encephalomyelitis (EAE) in mice. Using a spontaneously remitting EAE model, paralysis severity correlated inversely with both expression of synaptic proteins and the number of synapses in direct contact with the perikarya of motor neurons in spinal gray matter. In both remitting and non-remitting EAE models, paralysis severity also correlated inversely with sparcl1:sparc transcript and SPARCL1:SPARC protein ratios directly in lumbar spinal cord tissue. In vitro, astrocyte production of both SPARCL1 and SPARC was regulated by T cell-derived cytokines, causing dynamic modulation of the SPARCL1:SPARC expression ratio. Taken together, these data support a model whereby proinflammatory cytokines inhibit SPARCL1 and/or augment SPARC expression by astrocytes in spinal gray matter that, in turn, cause either transient or sustained synaptic retraction from lumbar spinal motor neurons thereby regulating hind limb paralysis during EAE. Ongoing studies seek ways to alter this SPARCL1:SPARC expression ratio in favor of synapse reformation/maintenance and thus help to modulate neurologic deficits during times of inflammation. This could identify new astrocyte-targeted therapies for diseases such as multiple sclerosis.
Keywords: EAE; SPARC; SPARCL1; astrocytes; multiple sclerosis; synaptic plasticity.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

