Functional connectivity in in vitro neuronal assemblies
- PMID: 26500505
- PMCID: PMC4595785
- DOI: 10.3389/fncir.2015.00057
Functional connectivity in in vitro neuronal assemblies
Abstract
Complex network topologies represent the necessary substrate to support complex brain functions. In this work, we reviewed in vitro neuronal networks coupled to Micro-Electrode Arrays (MEAs) as biological substrate. Networks of dissociated neurons developing in vitro and coupled to MEAs, represent a valid experimental model for studying the mechanisms governing the formation, organization and conservation of neuronal cell assemblies. In this review, we present some examples of the use of statistical Cluster Coefficients and Small World indices to infer topological rules underlying the dynamics exhibited by homogeneous and engineered neuronal networks.
Keywords: correlation; functional connectivity; graph theory; in vitro; micro-electrode arrays; neuronal network dynamics.
Figures

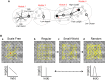
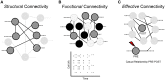
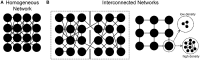

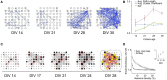

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

