Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus
- PMID: 26500509
- PMCID: PMC4593857
- DOI: 10.3389/fnana.2015.00130
Extra-neurohypophyseal axonal projections from individual vasopressin-containing magnocellular neurons in rat hypothalamus
Abstract
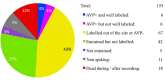
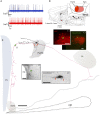
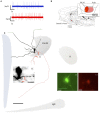
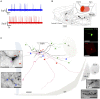
Conventional neuroanatomical, immunohistochemical techniques, and electrophysiological recording, as well as in vitro labeling methods may fail to detect long range extra-neurohypophyseal-projecting axons from vasopressin (AVP)-containing magnocellular neurons (magnocells) in the hypothalamic paraventricular nucleus (PVN). Here, we used in vivo extracellular recording, juxtacellular labeling, post-hoc anatomo-immunohistochemical analysis and camera lucida reconstruction to address this question. We demonstrate that all well-labeled AVP immunopositive neurons inside the PVN possess main axons joining the tract of Greving and multi-axon-like processes, as well as axonal collaterals branching very near to the somata, which project to extra-neurohypophyseal regions. The detected regions in this study include the medial and lateral preoptical area, suprachiasmatic nucleus (SCN), lateral habenula (LHb), medial and central amygdala and the conducting systems, such as stria medullaris, the fornix and the internal capsule. Expression of vesicular glutamate transporter 2 was observed in axon-collaterals. These results, in congruency with several previous reports in the literature, provided unequivocal evidence that AVP magnocells have an uncommon feature of possessing multiple axon-like processes emanating from somata or proximal dendrites. Furthermore, the long-range non-neurohypophyseal projections are more common than an "occasional" phenomenon as previously thought.
Keywords: axon collaterals; extra-hypothalamic projections; juxtacellular labeling; magnocellular neurosecretory system; paraventricular nucleus (PVN); vasopressin.
Figures


References
-
- Armstrong W. (2004). Hypothalamic supraoptic and paraventricular nuclei, in The Rat Nervous System, 3rd Edn., Chapter 15, ed Paxinos G. (Amsterdam: Elsevier; ), 369–388.
-
- Bargmann W., Scharrer E. (1951). The site of origin of the hormones of the posterior pituitary. Am. Sci. 39, 255–259. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

