Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation
- PMID: 26500660
- PMCID: PMC4593954
- DOI: 10.3389/fpls.2015.00805
Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation
Abstract
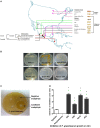
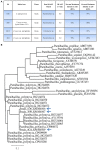
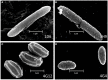
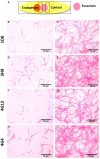
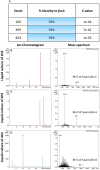
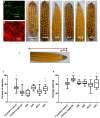
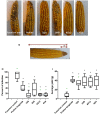
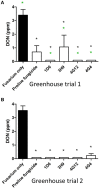
Wild maize (teosinte) has been reported to be less susceptible to pests than their modern maize (corn) relatives. Endophytes, defined as microbes that inhabit plants without causing disease, are known for their ability to antagonize plant pests and pathogens. We hypothesized that the wild relatives of modern maize may host endophytes that combat pathogens. Fusarium graminearum is the fungus that causes Gibberella Ear Rot (GER) in modern maize and produces the mycotoxin, deoxynivalenol (DON). In this study, 215 bacterial endophytes, previously isolated from diverse maize genotypes including wild teosintes, traditional landraces and modern varieties, were tested for their ability to antagonize F. graminearum in vitro. Candidate endophytes were then tested for their ability to suppress GER in modern maize in independent greenhouse trials. The results revealed that three candidate endophytes derived from wild teosintes were most potent in suppressing F. graminearum in vitro and GER in a modern maize hybrid. These wild teosinte endophytes could suppress a broad spectrum of fungal pathogens of modern crops in vitro. The teosinte endophytes also suppressed DON mycotoxin during storage to below acceptable safety threshold levels. A fourth, less robust anti-fungal strain was isolated from a modern maize hybrid. Three of the anti-fungal endophytes were predicted to be Paenibacillus polymyxa, along with one strain of Citrobacter. Microscopy studies suggested a fungicidal mode of action by all four strains. Molecular and biochemical studies showed that the P. polymyxa strains produced the previously characterized anti-Fusarium compound, fusaricidin. Our results suggest that the wild relatives of modern crops may serve as a valuable reservoir for endophytes in the ongoing fight against serious threats to modern agriculture. We discuss the possible impact of crop evolution and domestication on endophytes in the context of plant defense.
Keywords: Fusarium graminearum; Gibberella ear rot; Paenibacillus; Zea diploperennis; deoxynivalenol; endophyte; maize; parviglumis.
Figures

References
-
- Benrey B., Callejas A., Rios L., Oyama K., Denno R. F. (1998). The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol. Control 11, 130–140. 10.1006/bcon.1997.0590 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

