Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth
- PMID: 26500770
- PMCID: PMC4615872
- DOI: 10.1186/s40170-015-0138-0
Targeting mitochondrial complex I using BAY 87-2243 reduces melanoma tumor growth
Abstract
Background: Numerous studies have demonstrated that functional mitochondria are required for tumorigenesis, suggesting that mitochondrial oxidative phosphorylation (OXPHOS) might be a potential target for cancer therapy. In this study, we investigated the effects of BAY 87-2243, a small molecule that inhibits the first OXPHOS enzyme (complex I), in melanoma in vitro and in vivo.
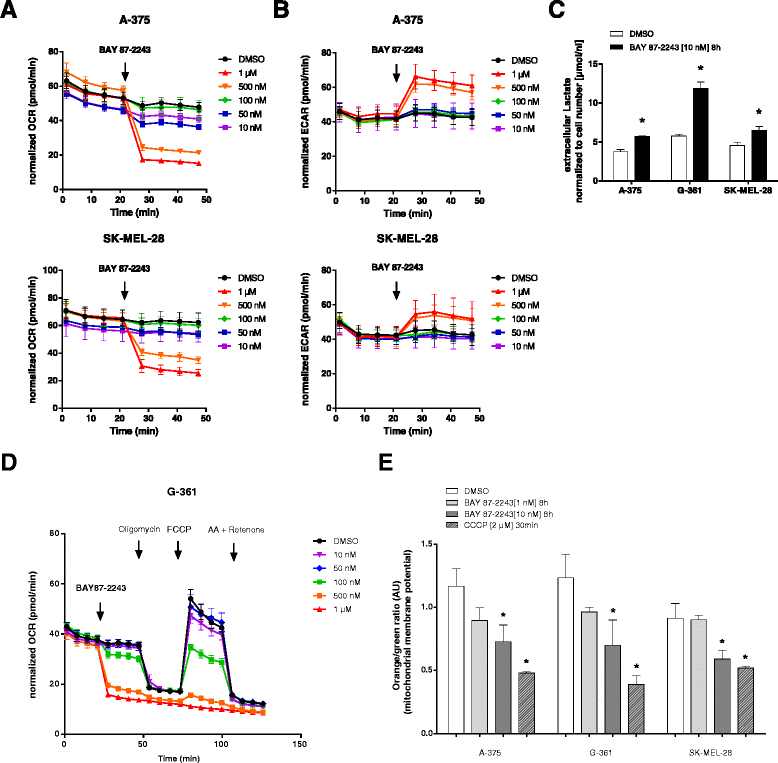
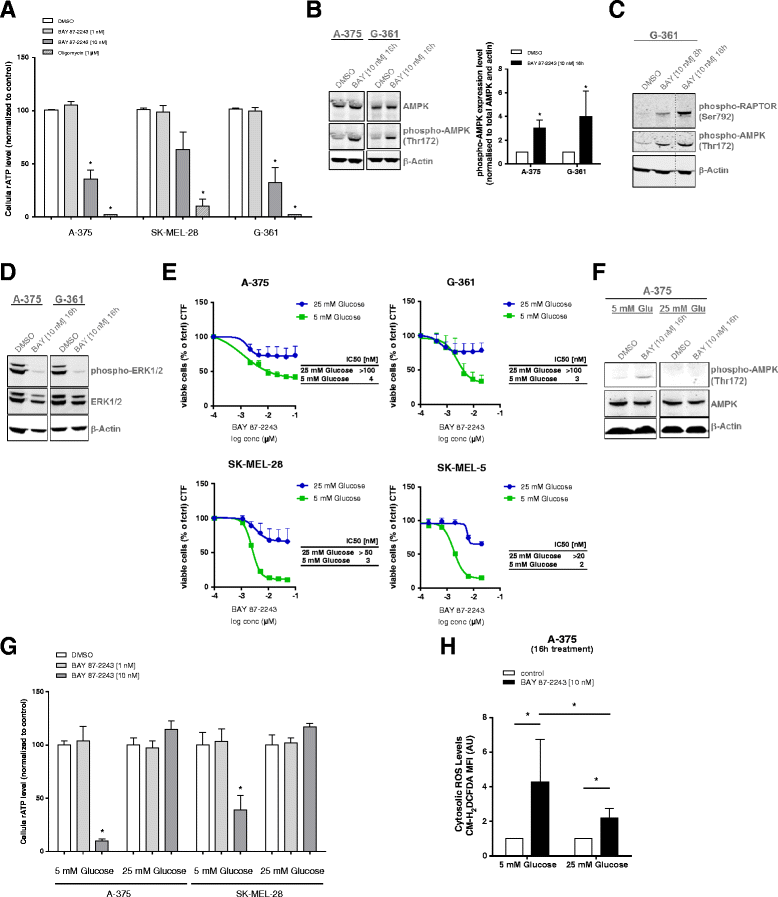
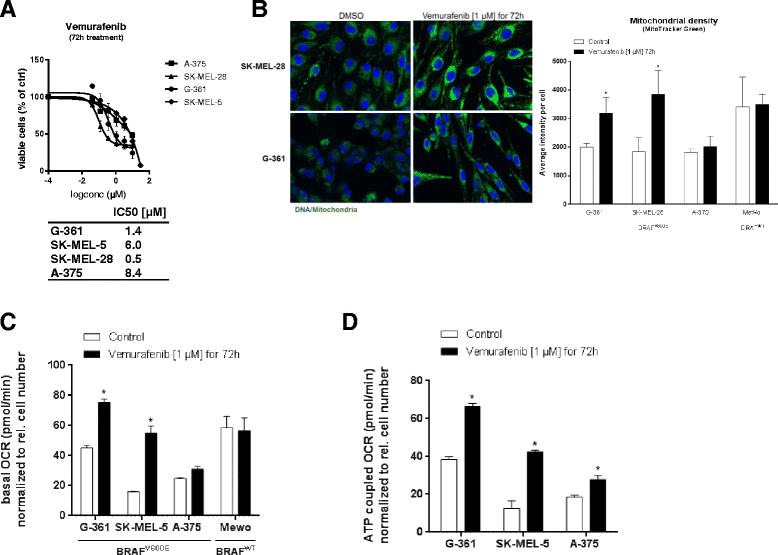
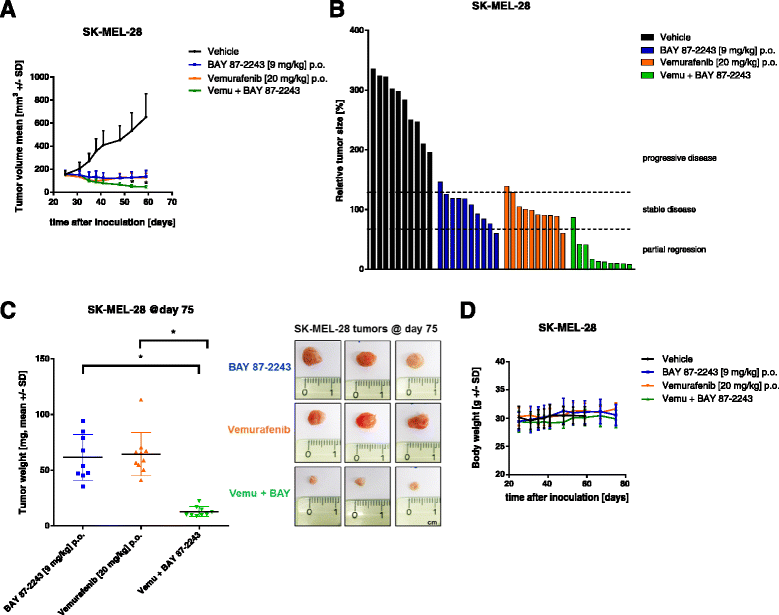
Results: BAY 87-2243 decreased mitochondrial oxygen consumption and induced partial depolarization of the mitochondrial membrane potential. This was associated with increased reactive oxygen species (ROS) levels, lowering of total cellular ATP levels, activation of AMP-activated protein kinase (AMPK), and reduced cell viability. The latter was rescued by the antioxidant vitamin E and high extracellular glucose levels (25 mM), indicating the involvement of ROS-induced cell death and a dependence on glycolysis for cell survival upon BAY 87-2243 treatment. BAY 87-2243 significantly reduced tumor growth in various BRAF mutant melanoma mouse xenografts and patient-derived melanoma mouse models. Furthermore, we provide evidence that inhibition of mutated BRAF using the specific small molecule inhibitor vemurafenib increased the OXPHOS dependency of BRAF mutant melanoma cells. As a consequence, the combination of both inhibitors augmented the anti-tumor effect of BAY 87-2243 in a BRAF mutant melanoma mouse xenograft model.
Conclusions: Taken together, our results suggest that complex I inhibition has potential clinical applications as a single agent in melanoma and also might be efficacious in combination with BRAF inhibitors in the treatment of patients with BRAF mutant melanoma.
Keywords: Anti-tumor efficacy; BRAF mutant melanoma; Cancer metabolism; Mitochondrial complex I; Oxidative phosphorylation (OXPHOS); Reactive oxygen species (ROS); Small molecule inhibitor.
Figures


References
-
- Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2(10):881–98. doi: 10.1158/2159-8290.CD-12-0345. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

