Investigating Reports of Complex Regional Pain Syndrome: An Analysis of HPV-16/18-Adjuvanted Vaccine Post-Licensure Data
- PMID: 26501109
- PMCID: PMC4587999
- DOI: 10.1016/j.ebiom.2015.07.003
Investigating Reports of Complex Regional Pain Syndrome: An Analysis of HPV-16/18-Adjuvanted Vaccine Post-Licensure Data
Abstract
Complex regional pain syndrome (CRPS) is a chronic pain disorder that typically follows trauma or surgery. Suspected CRPS reported after vaccination with human papillomavirus (HPV) vaccines led to temporary suspension of proactive recommendation of HPV vaccination in Japan. We investigated the potential CRPS signal in relation to HPV-16/18-adjuvanted vaccine (Cervarix®) by database review of CRPS cases with independent expert confirmation; a disproportionality analysis and analyses of temporality; an observed versus expected analysis using published background incidence rates; systematic reviews of aggregate safety data, and a literature review. The analysis included 17 case reports of CRPS: 10 from Japan (0.14/100,000 doses distributed) and seven from the United Kingdom (0.08/100,000). Five cases were considered by independent experts to be confirmed CRPS. Quantitative analyses did not suggest an association between CRPS and HPV-16/18-adjuvanted vaccine. Observed CRPS incidence after HPV-16/18 vaccination was statistically significantly below expected rates. Systematic database reviews using search terms varying in specificity and sensitivity did not identify new cases. No CRPS was reported during clinical development and no unexpected results found in the literature. There is not sufficient evidence to suggest an increased risk of developing CRPS following vaccination with HPV-16/18-adjuvanted vaccine. Post-licensure safety surveillance confirms the acceptable benefit-risk of HPV-16/18 vaccination.
Keywords: Chronic pain; Complex regional pain syndrome; Human papillomavirus vaccine; Safety.
Figures

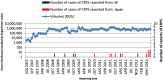
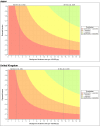

Comment in
-
HPV Vaccination and Complex Regional Pain Syndrome: Lack of Evidence.EBioMedicine. 2015 Aug 19;2(9):1014-5. doi: 10.1016/j.ebiom.2015.08.030. eCollection 2015 Sep. EBioMedicine. 2015. PMID: 26779563 Free PMC article. No abstract available.
References
-
- De Mos M., de Bruijn A.G.J., Huygen F.J.P.M., Dieleman J.P., Stricker B.H.C., Sturkenboom M.C.J.M. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. - PubMed
-
- De Rooij A.M., Florencia Gosso M., Haasnoot G.W. HLA-B62 and HLA-DQ8 are associated with complex regional pain syndrome with fixed dystonia. Pain. 2009;145:82–85. - PubMed
-
- De Rooij A.M., de Mos M., van Hilten J.J. Increased risk of complex regional pain syndrome in siblings of patients? J Pain Off J Am Pain Soc. 2009;10:1250–1255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

