Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids
- PMID: 26501191
- PMCID: PMC4753163
- DOI: 10.1038/nm.3973
Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids
Abstract
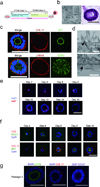
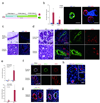
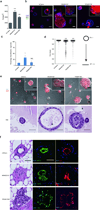
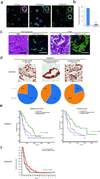
There are few in vitro models of exocrine pancreas development and primary human pancreatic adenocarcinoma (PDAC). We establish three-dimensional culture conditions to induce the differentiation of human pluripotent stem cells into exocrine progenitor organoids that form ductal and acinar structures in culture and in vivo. Expression of mutant KRAS or TP53 in progenitor organoids induces mutation-specific phenotypes in culture and in vivo. Expression of TP53(R175H) induces cytosolic SOX9 localization. In patient tumors bearing TP53 mutations, SOX9 was cytoplasmic and associated with mortality. We also define culture conditions for clonal generation of tumor organoids from freshly resected PDAC. Tumor organoids maintain the differentiation status, histoarchitecture and phenotypic heterogeneity of the primary tumor and retain patient-specific physiological changes, including hypoxia, oxygen consumption, epigenetic marks and differences in sensitivity to inhibition of the histone methyltransferase EZH2. Thus, pancreatic progenitor organoids and tumor organoids can be used to model PDAC and for drug screening to identify precision therapy strategies.
Figures

References
-
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Postgraduate Medical Journal. 2008;84:478–497. - PubMed
-
- Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods in enzymology. 2006;407:703–710. - PubMed
Methods References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

