Rare Variants in the Functional Domains of Complement Factor H Are Associated With Age-Related Macular Degeneration
- PMID: 26501415
- PMCID: PMC4627248
- DOI: 10.1167/iovs.15-17432
Rare Variants in the Functional Domains of Complement Factor H Are Associated With Age-Related Macular Degeneration
Abstract
Purpose: Age-related macular degeneration (AMD) has a substantial genetic risk component, as evidenced by the risk from common genetic variants uncovered in the first genome-wide association studies. More recently, it has become apparent that rare genetic variants also play an independent role in AMD risk. We sought to determine if rare variants in complement factor H (CFH) played a role in AMD risk.
Methods: We had previously collected DNA from a large population of patients with advanced age-related macular degeneration (A-AMD) and controls for targeted deep sequencing of candidate AMD risk genes. In this analysis, we tested for an increased burden of rare variants in CFH in 1665 cases and 752 controls from this cohort.
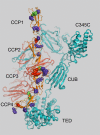
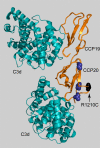
Results: We identified 65 missense, nonsense, or splice-site mutations with a minor allele frequency ≤ 1%. Rare variants with minor allele frequency ≤ 1% (odds ratio [OR] = 1.5, P = 4.4 × 10⁻²), 0.5% (OR = 1.6, P = 2.6 × 10⁻²), and all singletons (OR = 2.3, P = 3.3 × 10⁻²) were enriched in A-AMD cases. Moreover, we observed loss-of-function rare variants (nonsense, splice-site, and loss of a conserved cysteine) in 10 cases and serum levels of FH were decreased in all 5 with an available sample (haploinsufficiency). Further, rare variants in the major functional domains of CFH were increased in cases (OR = 3.2; P = 1.4 × 10⁻³) and the magnitude of the effect correlated with the disruptive nature of the variant, location in an active site, and inversely with minor allele frequency.
Conclusions: In this large A-AMD cohort, rare variants in the CFH gene were enriched and tended to be located in functional sites or led to low serum levels. These data, combined with those indicating a similar, but even more striking, increase in rare variants found in CFI, strongly implicate complement activation in A-AMD etiopathogenesis as CFH and CFI interact to inhibit the alternative pathway.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- U01 HG007690/HG/NHGRI NIH HHS/United States
- 1U01HG007690-01/HG/NHGRI NIH HHS/United States
- 1R01AR063759-01A1/AR/NIAMS NIH HHS/United States
- R01-EY11309/EY/NEI NIH HHS/United States
- R01-AI041592/AI/NIAID NIH HHS/United States
- U01 GM092691/GM/NIGMS NIH HHS/United States
- P30 AR048335/AR/NIAMS NIH HHS/United States
- 5U01GM092691-04/GM/NIGMS NIH HHS/United States
- R01 EY011309/EY/NEI NIH HHS/United States
- P30 AR48335/AR/NIAMS NIH HHS/United States
- F30 HL103072/HL/NHLBI NIH HHS/United States
- R01 AR063759/AR/NIAMS NIH HHS/United States
- U54 HL112303/HL/NHLBI NIH HHS/United States
- F30HL103072/HL/NHLBI NIH HHS/United States
- R01 AI041592/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

