GDF10 is a signal for axonal sprouting and functional recovery after stroke
- PMID: 26502261
- PMCID: PMC4790086
- DOI: 10.1038/nn.4146
GDF10 is a signal for axonal sprouting and functional recovery after stroke
Abstract
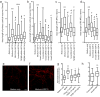
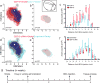
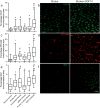
Stroke produces a limited process of neural repair. Axonal sprouting in cortex adjacent to the infarct is part of this recovery process, but the signal that initiates axonal sprouting is not known. Growth and differentiation factor 10 (GDF10) is induced in peri-infarct neurons in mice, non-human primates and humans. GDF10 promotes axonal outgrowth in vitro in mouse, rat and human neurons through TGFβRI and TGFβRII signaling. Using pharmacogenetic gain- and loss-of-function studies, we found that GDF10 produced axonal sprouting and enhanced functional recovery after stroke; knocking down GDF10 blocked axonal sprouting and reduced recovery. RNA sequencing from peri-infarct cortical neurons revealed that GDF10 downregulated PTEN, upregulated PI3 kinase signaling and induced specific axonal guidance molecules. Using unsupervised genome-wide association analysis of the GDF10 transcriptome, we found that it was not related to neurodevelopment, but may partially overlap with other CNS injury patterns. Thus, GDF10 is a stroke-induced signal for axonal sprouting and functional recovery.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

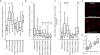
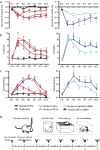
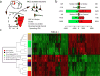
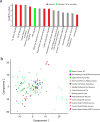
Comment in
-
Stroke: GDF10 spurs on axonal sprouting after stroke.Nat Rev Drug Discov. 2016 Jan;15(1):16. doi: 10.1038/nrd.2015.27. Nat Rev Drug Discov. 2016. PMID: 26718278 No abstract available.
References
-
- Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29:1719–34. - PMC - PubMed
-
- Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke. 2014;45:1077–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

