Induction of interferon-λ contributes to TLR3 and RIG-I activation-mediated inhibition of herpes simplex virus type 2 replication in human cervical epithelial cells
- PMID: 26502803
- PMCID: PMC4664393
- DOI: 10.1093/molehr/gav058
Induction of interferon-λ contributes to TLR3 and RIG-I activation-mediated inhibition of herpes simplex virus type 2 replication in human cervical epithelial cells
Abstract
Study hypothesis: Is it possible to immunologically activate human cervical epithelial cells to produce antiviral factors that inhibit herpes simplex virus type 2 (HSV-2) replication?
Study finding: Our results indicate that human cervical epithelial cells possess a functional TLR3/RIG-I signaling system, the activation of which can mount an Interferon-λ (IFN-λ)-mediated anti-HSV-2 response.
What is known already: There is limited information about the role of cervical epithelial cells in genital innate immunity against HSV-2 infection.
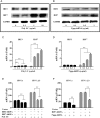
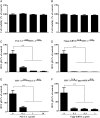
Study design, samples/materials, methods: We examined the expression of toll-like receptors (TLRs) and retinoic acid-inducible I (RIG-I) in End1/E6E7 cells by real-time PCR. The IFN-λ induced by TLR3 and RIG-I activation of End1/E6E7 cells was also examined by real-time PCR and ELISA. HSV-2 infection of End1/E6E7 cells was evaluated by the real-time PCR detection of HSV-2 gD expression. The antibody to IL-10Rβ was used to determine whether IFN-λ contributes to TLR3/RIG-I mediated HSV-2 inhibition. Expression of interferon regulatory factor 3 (IRF3), IRF7, IFN-stimulated gene 56 (ISG56), 2'-5'-oligoadenylate synthetase I (OAS-1) and myxovirus resistance A (MxA) were determined by the real-time PCR and western blot. End1/E6E7 cells were transfected with shRNA to knockdown the IRF3, IRF7 or RIG-I expression. Student's t-test and post Newman-Keuls test were used to analyze stabilized differences in the immunological parameters above between TLR3/RIG-I-activated cells and control cells.
Main results and the role of chance: Human cervical epithelial cells expressed functional TLR3 and RIG-I, which could be activated by poly I:C and 5'ppp double-strand RNAs (5'ppp dsRNA), resulting in the induction of endogenous interferon lambda (IFN-λ). The induced IFN-λ contributed to TLR3/RIG-I-mediated inhibition of HSV-2 replication in human cervical epithelial cells, as an antibody to IL-10Rβ, an IFN-λ receptor subunit, could compromise TLR3/RIG-I-mediated inhibition of HSV-2. Further studies showed that TLR3/RIG-I signaling in the cervical epithelial cells by dsRNA induced the expression of the IFN-stimulated genes (ISGs), ISG56, 2'-5'-oligoadenylate synthetase I (OAS-1) and myxovirus resistance A (MxA), the key antiviral elements in the IFN signaling pathway. In addition, we observed that the topical treatment of genital mucosa with poly I:C could protect mice from genital HSV-2 infection.
Limitations, reasons for caution: Future prospective studies with primary cells and suitable animal models are needed in order to confirm these outcomes.
Wider implications of the findings: The findings provide direct and compelling evidence that there is intracellular expression and regulation of IFN-λ in human cervical epithelial cells, which may have a key role in the innate genital protection against viral infections.
Large scale data: Not applicable.
Study funding and competing interests: This work was supported by the National Natural Science Foundation of China (81301428 to L.Z. and 81271334 to W.-Z.H.), the Fundamental Research Funds for the Central Universities (2042015kf0188 to L.Z.), the China Postdoctoral Science Foundation (2013M531745 to L.Z.), the Development Program of China ('973', 2012CB518900 to W.-Z.H.) from the Ministry of Science and Technology of the People's Republic of China, grants (DA12815 and DA022177 to W.-Z.H.) from the National Institute on Drug Abuse (NIDA) and the open project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research (WDCM005 to M.S.). The authors declare no competing financial interests.
Keywords: Toll-like receptor 3; herpes simplex virus type 2; human cervical epithelial cells; interferon-stimulated genes; interferon-λ; retinoic acid-inducible gene I.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001;413:732–738. - PubMed
-
- Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors 2009;35:82–87. - PubMed
-
- Ashkar AA, Yao XD, Gill N, Sajic D, Patrick AJ, Rosenthal KL. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J Infect Dis 2004;190:1841–1849. - PubMed
-
- Azulay-Debby H, Edry E, Melamed D. CpG DNA stimulates autoreactive immature B cells in the bone marrow. Eur J Immunol 2007;37:1463–1475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

