Involvement of an Skp-Like Protein, PGN_0300, in the Type IX Secretion System of Porphyromonas gingivalis
- PMID: 26502912
- PMCID: PMC4693996
- DOI: 10.1128/IAI.01308-15
Involvement of an Skp-Like Protein, PGN_0300, in the Type IX Secretion System of Porphyromonas gingivalis
Abstract
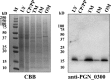
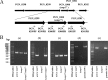
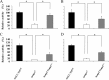
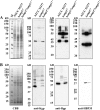
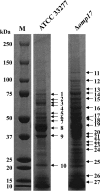
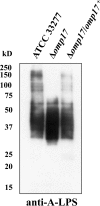
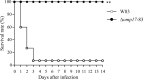
The oral Gram-negative anaerobic bacterium Porphyromonas gingivalis is an important pathogen involved in chronic periodontitis. Among its virulence factors, the major extracellular proteinases, Arg-gingipain and Lys-gingipain, are of interest given their abilities to degrade host proteins and process other virulence factors. Gingipains possess C-terminal domains (CTDs) and are translocated to the cell surface or into the extracellular milieu by the type IX secretion system (T9SS). Gingipains contribute to the colonial pigmentation of the bacterium on blood agar. In this study, Omp17, the PGN_0300 gene product, was found in the outer membrane fraction. A mutant lacking Omp17 did not show pigmentation on blood agar and showed reduced proteolytic activity of the gingipains. CTD-containing proteins were released from bacterial cells without cleavage of the CTDs in the omp17 mutant. Although synthesis of the anionic polysaccharide (A-LPS) was not affected in the omp17 mutant, the processing of and A-LPS modification of CTD-containing proteins was defective. PorU, a C-terminal signal peptidase that cleaves the CTDs of other CTD-containing proteins, was not detected in any membrane fraction of the omp17 mutant, suggesting that the defective maturation of CTD-containing proteins by impairment of Omp17 is partly due to loss of function of PorU. In the mouse subcutaneous infection experiment, the omp17 mutant was less virulent than the wild type. These results suggested that Omp17 is involved in P. gingivalis virulence.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
PGN_0297 is an essential component of the type IX secretion system (T9SS) in Porphyromonas gingivalis: Tn-seq analysis for exhaustive identification of T9SS-related genes.Microbiol Immunol. 2019 Jan;63(1):11-20. doi: 10.1111/1348-0421.12665. Microbiol Immunol. 2019. PMID: 30599082 Free PMC article.
-
PorZ, an Essential Component of the Type IX Secretion System of Porphyromonas gingivalis, Delivers Anionic Lipopolysaccharide to the PorU Sortase for Transpeptidase Processing of T9SS Cargo Proteins.mBio. 2021 Feb 23;12(1):e02262-20. doi: 10.1128/mBio.02262-20. mBio. 2021. PMID: 33622730 Free PMC article.
-
Methods for Functional Characterization of the Type IX Secretion System of Porphyromonas gingivalis.Methods Mol Biol. 2021;2210:123-133. doi: 10.1007/978-1-0716-0939-2_12. Methods Mol Biol. 2021. PMID: 32815133
-
Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility.J Periodontal Res. 2015 Feb;50(1):1-8. doi: 10.1111/jre.12255. Epub 2014 Dec 27. J Periodontal Res. 2015. PMID: 25546073 Free PMC article. Review.
-
Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors.Curr Protein Pept Sci. 2003 Dec;4(6):389-95. doi: 10.2174/1389203033486983. Curr Protein Pept Sci. 2003. PMID: 14683425 Review.
Cited by
-
Gingimaps: Protein Localization in the Oral Pathogen Porphyromonas gingivalis.Microbiol Mol Biol Rev. 2020 Jan 2;84(1):e00032-19. doi: 10.1128/MMBR.00032-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31896547 Free PMC article. Review.
-
Characterization of the Porphyromonas gingivalis Type IX Secretion Trans-envelope PorKLMNP Core Complex.J Biol Chem. 2017 Feb 24;292(8):3252-3261. doi: 10.1074/jbc.M116.765081. Epub 2017 Jan 5. J Biol Chem. 2017. PMID: 28057754 Free PMC article.
-
Research progress on two-component signal transduction systems in Porphyromonas gingivalis.Hua Xi Kou Qiang Yi Xue Za Zhi. 2021 Feb 1;39(1):88-93. doi: 10.7518/hxkq.2021.01.013. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021. PMID: 33723942 Free PMC article. Review. Chinese, English.
-
Riemerella anatipestifer GldM is required for bacterial gliding motility, protein secretion, and virulence.Vet Res. 2019 Jun 4;50(1):43. doi: 10.1186/s13567-019-0660-0. Vet Res. 2019. PMID: 31164171 Free PMC article.
-
Structural and functional probing of PorZ, an essential bacterial surface component of the type-IX secretion system of human oral-microbiomic Porphyromonas gingivalis.Sci Rep. 2016 Nov 24;6:37708. doi: 10.1038/srep37708. Sci Rep. 2016. PMID: 27883039 Free PMC article.
References
-
- Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. 1995. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J Biol Chem 270:23619–23626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

