Thalamic control of sensory selection in divided attention
- PMID: 26503050
- PMCID: PMC4626291
- DOI: 10.1038/nature15398
Thalamic control of sensory selection in divided attention
Abstract
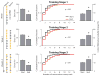
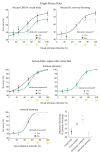
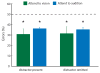
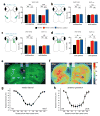
How the brain selects appropriate sensory inputs and suppresses distractors is unknown. Given the well-established role of the prefrontal cortex (PFC) in executive function, its interactions with sensory cortical areas during attention have been hypothesized to control sensory selection. To test this idea and, more generally, dissect the circuits underlying sensory selection, we developed a cross-modal divided-attention task in mice that allowed genetic access to this cognitive process. By optogenetically perturbing PFC function in a temporally precise window, the ability of mice to select appropriately between conflicting visual and auditory stimuli was diminished. Equivalent sensory thalamocortical manipulations showed that behaviour was causally dependent on PFC interactions with the sensory thalamus, not sensory cortex. Consistent with this notion, we found neurons of the visual thalamic reticular nucleus (visTRN) to exhibit PFC-dependent changes in firing rate predictive of the modality selected. visTRN activity was causal to performance as confirmed by bidirectional optogenetic manipulations of this subnetwork. Using a combination of electrophysiology and intracellular chloride photometry, we demonstrated that visTRN dynamically controls visual thalamic gain through feedforward inhibition. Our experiments introduce a new subcortical model of sensory selection, in which the PFC biases thalamic reticular subnetworks to control thalamic sensory gain, selecting appropriate inputs for further processing.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

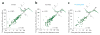
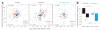
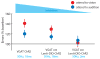
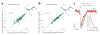



Comment in
-
A Subcortical Pathway for Rapid, Goal-Driven, Attentional Filtering.Trends Neurosci. 2016 Feb;39(2):49-51. doi: 10.1016/j.tins.2015.12.003. Epub 2015 Dec 30. Trends Neurosci. 2016. PMID: 26743499
References
-
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. - PubMed
-
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–2. - PubMed
-
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–23. - PubMed
-
- Rodgers CC, DeWeese MR. Neural correlates of task switching in prefrontal cortex and primary auditory cortex in a novel stimulus selection task for rodents. Neuron. 2014;82:1157–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

