Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle
- PMID: 26503074
- PMCID: PMC5023701
- DOI: 10.1113/JP270659
Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle
Abstract
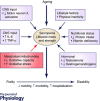
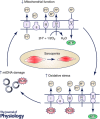
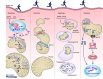
Mitochondria are negatively affected by ageing leading to their inability to adapt to higher levels of oxidative stress and this ultimately contributes to the systemic loss of muscle mass and function termed sarcopenia. Since mitochondria are central mediators of muscle health, they have become highly sought-after targets of physiological and pharmacological interventions. Exercise is the only known strategy to combat sarcopenia and this is largely mediated through improvements in mitochondrial plasticity. More recently a critical role for mitochondrial turnover in preserving muscle has been postulated. Specifically, cellular pathways responsible for the regulation of mitochondrial turnover including biogenesis, dynamics and autophagy may become dysregulated during ageing resulting in the reduced clearance and accumulation of damaged organelles within the cell. When mitochondrial quality is compromised and homeostasis is not re-established, myonuclear cell death is activated and muscle atrophy ensues. In contrast, acute and chronic exercise attenuates these deficits, restoring mitochondrial turnover and promoting a healthier mitochondrial pool that leads to the preservation of muscle. Additionally, the magnitude of these exercise-induced mitochondrial adaptations is currently debated with several studies reporting a lower adaptability of old muscle relative to young, but the processes responsible for this diminished training response are unclear. Based on these observations, understanding the molecular details of how advancing age and exercise influence mitochondria in older muscle will provide invaluable insight into the development of exercise protocols that will maximize beneficial adaptations in the elderly. This information will also be imperative for future research exploring pharmacological targets of mitochondrial plasticity.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
References
-
- Ades PA, Ballor DL, Ashikaga T, Utton JL & Nair KS (1996). Weight training improves walking endurance in healthy elderly persons. Ann Intern Med 124, 568–572. - PubMed
-
- Adhihetty PJ, O'Leary MF & Hood DA (2008). Mitochondria in skeletal muscle: adaptable rheostats of apoptotic susceptibility. Exerc Sport Sci Rev 36, 116–121. - PubMed
-
- Barrientos A, Casademont J, Rotig A, Miro O, Urbano‐Marquez A, Rustin P & Cardellach F (1996). Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun 229, 536–539. - PubMed
-
- Beregi E, Regius O, Huttl T & Gobl Z (1988). Age‐related changes in the skeletal muscle cells. Z Gerontol 21, 83–86. - PubMed
-
- Betik AC, Thomas MM, Wright KJ, Riel CD & Hepple RT (2009). Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol 297, R744–755. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

