Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light
- PMID: 26503125
- PMCID: PMC4621539
- DOI: 10.1038/srep15718
Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light
Abstract
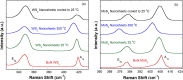
Exfoliated transition metal dichalcogenides (TMDs) such as WS2 and MoS2 have shown exciting potential for energy storage, catalysis and optoelectronics. So far, solution based methods for scalable production of few-layer TMDs usually involve the use of organic solvents or dangerous chemicals. Here, we report an eco-friendly method for facile synthesis of few-layer WS2 and MoS2 nanosheets using dilute aqueous solution of household detergent. Short time sonication of varying amount of bulk samples in soapy water was used to scale up the production of nanosheets. Thermal stability, optical absorption and Raman spectra of as-synthesized WS2 and MoS2 nanosheets are in close agreement with those from other synthesis techniques. Efficient photocatalytic activity of TMDs nanosheets was demonstrated by decomposing Brilliant Green dye in aqueous solution under visible light irradiation. Our study shows the great potential of TMDs nanosheets for environmental remediation by degrading toxic industrial chemicals in wastewater using sunlight.
Figures




References
-
- Wang Q. H., Kalantar-Zadeh K., Kis A., Coleman J. N. & Strano M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012). - PubMed
-
- Kim S. et al. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 3, 1011 (2012). - PubMed
-
- Matte H. S. S. R. et al. MoS2 and WS2 Analogues of Graphene. Angew. Chem. 122, 4153–4156 (2010). - PubMed
-
- Mak K. F., Lee C., Hone J., Shan J. & Heinz T. F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 105, 136805 (2010). - PubMed
-
- Chhowalla M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

