Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group
- PMID: 26503200
- PMCID: PMC5070550
- DOI: 10.1200/JCO.2015.63.4048
Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group
Abstract
Purpose: Patients with metastatic rhabdomyosarcoma (RMS), except those younger than 10 years with embryonal RMS, have an estimated long-term event-free survival (EFS) of less than 20%. The main goal of this study was to improve outcome of patients with metastatic RMS by dose intensification with interval compression, use of the most active agents determined in phase II window studies, and use of irinotecan as a radiation sensitizer.
Patients and methods: Patients with metastatic RMS received 54 weeks of therapy: blocks of therapy with vincristine/irinotecan (weeks 1 to 6, 20 to 25, and 47 to 52), interval compression with vincristine/doxorubicin/cyclophosphamide alternating with etoposide/ifosfamide (weeks 7 to 19 and 26 to 34), and vincristine/dactinomycin/cyclophosphamide (weeks 38 to 46). Radiation therapy occurred at weeks 20 to 25 (primary) but was also permitted at weeks 1 to 6 (for intracranial or paraspinal extension) and weeks 47 to 52 (for extensive metastatic sites).
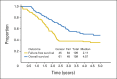
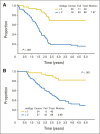
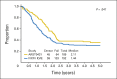
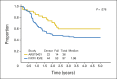
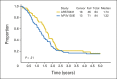
Results: One hundred nine eligible patients were enrolled, with a median follow-up of surviving patients of 3.8 years (3-year EFS for all patients, 38% [95% CI, 29% to 48%]; survival, 56% [95% CI, 46% to 66%]). Patients with one or no Oberlin risk factor (age > 10 years or < 1 year, unfavorable primary site of disease, ≥ three metastatic sites, and bone or bone marrow involvement) had a 3-year EFS of 69% (95% CI, 52% to 82%); high-risk patients with two or more risk factors had a 3-year EFS of 20% (95% CI, 11% to 30%). Toxicity was similar to that on prior RMS studies.
Conclusion: Patients with metastatic RMS with one or no Oberlin risk factor had an improved 3-year EFS of 69% on ARST0431 compared with an historical cohort from pooled European and US studies; those with two or more risk factors have a dismal prognosis, and new approaches are needed for this very-high-risk group.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
Comment in
-
Metastatic Rhabdomyosarcoma: Still Room for Improvement.J Clin Oncol. 2016 Jan 10;34(2):105-6. doi: 10.1200/JCO.2015.64.3395. Epub 2015 Nov 16. J Clin Oncol. 2016. PMID: 26573072 No abstract available.
References
-
- Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. - PubMed
-
- Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. - PubMed
-
- Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. - PubMed
-
- Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. - PubMed
-
- Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

