Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2
- PMID: 26503204
- PMCID: PMC5070556
- DOI: 10.1200/JCO.2014.60.1971
Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2
Erratum in
Abstract
Purpose: To explore the genetic landscape of tumors from patients enrolled on the BOLERO-2 trial to identify potential correlations between genetic alterations and efficacy of everolimus treatment. The BOLERO-2 trial has previously demonstrated that the addition of everolimus to exemestane prolonged progression-free survival by more than twofold in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer previously treated with nonsteroidal aromatase inhibitors.
Patients and methods: Next-generation sequencing was used to analyze genetic status of cancer-related genes in 302 archival tumor specimens from patients representative of the BOLERO-2 study population. Correlations between the most common somatic alterations and degree of chromosomal instability, and treatment effect of everolimus were investigated.
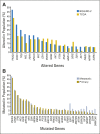
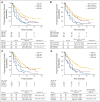
Results: Progression-free survival benefit with everolimus was maintained regardless of alteration status of PIK3CA, FGFR1, and CCND1 or the pathways of which they are components. However, quantitative differences in everolimus benefit were observed between patient subgroups defined by the exon-specific mutations in PIK3CA (exon 20 v 9) or by different degrees of chromosomal instability in the tumor tissues.
Conclusion: The data from this exploratory analysis suggest that the efficacy of everolimus was largely independent of the most commonly altered genes or pathways in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer. The potential impact of chromosomal instabilities and low-frequency genetic alterations on everolimus efficacy warrants further investigation.
Trial registration: ClinicalTrials.gov NCT00863655.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
Comment in
-
Targeted Combinations for Hormone Receptor-Positive Advanced Breast Cancer: Who Benefits?J Clin Oncol. 2016 Feb 10;34(5):393-5. doi: 10.1200/JCO.2015.64.0771. Epub 2015 Dec 23. J Clin Oncol. 2016. PMID: 26700117 No abstract available.
References
-
- Barbareschi M, Buttitta F, Felicioni L, et al. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007;13:6064–6069. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

