Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer
- PMID: 26503259
- PMCID: PMC4698020
- DOI: 10.1007/s11605-015-3000-3
Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer
Abstract
Introduction: Oxaliplatin is part of pancreatic cancer therapy in the FOLFIRINOX or GEMOX/XELOX regimen. DNA damage repair is one of the factors responsible for oxaliplatin resistance that eventually develops in this cancer. Triptolide/Minnelide has been shown to be effective against pancreatic cancer in preclinical trials. In this study, we evaluated the efficacy of combination of triptolide and oxaliplatin against pancreatic cancer.
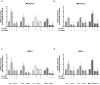
Methods: Highly aggressive pancreatic cancer cells (MIA PaCa-2 and PANC-1) were treated with oxaliplatin (0-10 μM), low-dose triptolide (50 nM), or a combination of both for 24-48 h. Cell viability, apoptosis, and DNA damage were evaluated by appropriate methods. Nucleotide excision repair pathway components were quantitated using qPCR and Western blot. Combination of low doses of Minnelide and oxaliplatin was tested in an orthotopic murine model of pancreatic cancer.
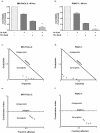
Results: Proliferation of pancreatic cancer cells was markedly inhibited by combination treatment. Triptolide potentiated apoptotic cell death induced by oxaliplatin and sensitized cancer cells towards oxaliplatin-induced DNA damage by suppressing the oxaliplatin-induced DNA damage repair pathway. Combination of low doses of Minnelide and oxaliplatin inhibited tumor progression by inducing significant apoptotic cell death in these tumors.
Conclusions: Combination of low doses of Minnelide and oxaliplatin has immense potential to emerge as a novel therapeutic strategy against pancreatic cancer.
Keywords: DNA damage pancreatic cancer; Minnelide; NER; Oxaliplatin; Triptolide.
Figures

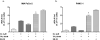

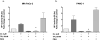

Similar articles
-
Triptolide enhances the tumoricidal activity of TRAIL against renal cell carcinoma.FEBS J. 2015 Dec;282(24):4747-4765. doi: 10.1111/febs.13532. Epub 2015 Oct 18. FEBS J. 2015. PMID: 26426449 Free PMC article.
-
Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer.PLoS One. 2017 Feb 13;12(2):e0171827. doi: 10.1371/journal.pone.0171827. eCollection 2017. PLoS One. 2017. PMID: 28192510 Free PMC article.
-
Triptolide as a novel agent in pancreatic cancer: the validation using patient derived pancreatic tumor cell line.BMC Cancer. 2018 Nov 12;18(1):1103. doi: 10.1186/s12885-018-4995-0. BMC Cancer. 2018. PMID: 30419860 Free PMC article.
-
Triptolide and Its Derivatives as Cancer Therapies.Trends Pharmacol Sci. 2019 May;40(5):327-341. doi: 10.1016/j.tips.2019.03.002. Epub 2019 Apr 8. Trends Pharmacol Sci. 2019. PMID: 30975442 Review.
-
Minnelide, a novel drug for pancreatic and liver cancer.Pancreatology. 2015 Jul;15(4 Suppl):S39-43. doi: 10.1016/j.pan.2015.05.472. Epub 2015 Jun 18. Pancreatology. 2015. PMID: 26122306 Free PMC article. Review.
Cited by
-
Natural biomolecules and derivatives as anticancer immunomodulatory agents.Front Immunol. 2023 Jan 9;13:1070367. doi: 10.3389/fimmu.2022.1070367. eCollection 2022. Front Immunol. 2023. PMID: 36700235 Free PMC article. Review.
-
Advanced Nanoengineering Approach for Target-Specific, Spatiotemporal, and Ratiometric Delivery of Gemcitabine-Cisplatin Combination for Improved Therapeutic Outcome in Pancreatic Cancer.Small. 2022 Jan;18(2):e2104449. doi: 10.1002/smll.202104449. Epub 2021 Nov 10. Small. 2022. PMID: 34758094 Free PMC article.
-
"Heat shock protein 70 in pancreatic diseases: Friend or foe".J Surg Oncol. 2017 Jul;116(1):114-122. doi: 10.1002/jso.24653. Epub 2017 May 22. J Surg Oncol. 2017. PMID: 28543919 Free PMC article. Review.
-
Utilization of natural products in diverse pathogeneses of diseases associated with single or double DNA strand damage repair.Chin Med. 2025 Apr 7;20(1):46. doi: 10.1186/s13020-025-01089-y. Chin Med. 2025. PMID: 40197523 Free PMC article. Review.
-
Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment.MedComm (2020). 2021 Jun 10;2(3):315-340. doi: 10.1002/mco2.55. eCollection 2021 Sep. MedComm (2020). 2021. PMID: 34766149 Free PMC article. Review.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. - PubMed
-
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

