Corticospinal axons make direct synaptic connections with spinal motoneurons innervating forearm muscles early during postnatal development in the rat
- PMID: 26503304
- PMCID: PMC4704511
- DOI: 10.1113/JP270885
Corticospinal axons make direct synaptic connections with spinal motoneurons innervating forearm muscles early during postnatal development in the rat
Abstract
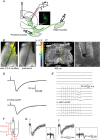
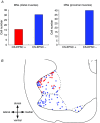
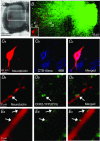
Direct connections between corticospinal (CS) axons and motoneurons (MNs) appear to be present only in higher primates, where they are essential for discrete movement of the digits. Their presence in adult rodents was once claimed but is now questioned. We report that MNs innervating forearm muscles in infant rats receive monosynaptic input from CS axons, but MNs innervating proximal muscles do not, which is a pattern similar to that in primates. Our experiments were carefully designed to show monosynaptic connections. This entailed selective electrical and optogenetic stimulation of CS axons and recording from MNs identified by retrograde labelling from innervated muscles. Morphological evidence was also obtained for rigorous identification of CS axons and MNs. These connections would be transient and would regress later during development. These results shed light on the development and evolution of direct CS-MN connections, which serve as the basis for dexterity in humans. Recent evidence suggests there is no direct connection between corticospinal (CS) axons and spinal motoneurons (MNs) in adult rodents. We previously showed that CS synapses are present throughout the spinal cord for a time, but are eliminated from the ventral horn during development in rodents. This raises the possibility that CS axons transiently make direct connections with MNs located in the ventral horn of the spinal cord. This was tested in the present study. Using cervical cord slices prepared from rats on postnatal days (P) 7-9, CS axons were stimulated and whole cell recordings were made from MNs retrogradely labelled with fluorescent cholera toxin B subunit (CTB) injected into selected groups of muscles. To selectively activate CS axons, electrical stimulation was carefully limited to the CS tract. In addition we employed optogenetic stimulation after injecting an adeno-associated virus vector encoding channelrhodopsin-2 (ChR2) into the sensorimotor cortex on P0. We were then able to record monosynaptic excitatory postsynaptic currents from MNs innervating forearm muscles, but not from those innervating proximal muscles. We also showed close contacts between CTB-labelled MNs and CS axons labelled through introduction of fluorescent protein-conjugated synaptophysin or the ChR2 expression system. We confirmed that some of these contacts colocalized with postsynaptic density protein 95 in their partner dendrites. It is intriguing from both phylogenetic and ontogenetic viewpoints that direct and putatively transient CS-MN connections were found only on MNs innervating the forearm muscles in infant rats, as this is analogous to the connection pattern seen in adult primates.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



Similar articles
-
Higher primate-like direct corticomotoneuronal connections are transiently formed in a juvenile subprimate mammal.Sci Rep. 2018 Nov 8;8(1):16536. doi: 10.1038/s41598-018-34961-z. Sci Rep. 2018. PMID: 30410053 Free PMC article.
-
An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses.Exp Brain Res. 2003 Apr;149(4):458-69. doi: 10.1007/s00221-003-1393-9. Epub 2003 Feb 21. Exp Brain Res. 2003. PMID: 12677326
-
Corticospinal tract development and spinal cord innervation differ between cervical and lumbar targets.J Neurosci. 2015 Jan 21;35(3):1181-91. doi: 10.1523/JNEUROSCI.2842-13.2015. J Neurosci. 2015. PMID: 25609632 Free PMC article.
-
The Florey lecture, 1987. Corticomotoneuronal projections: synaptic events related to skilled movement.Proc R Soc Lond B Biol Sci. 1987 Jul 22;231(1263):147-68. doi: 10.1098/rspb.1987.0039. Proc R Soc Lond B Biol Sci. 1987. PMID: 2889209 Review.
-
Physiological basis of motor effects of a transient stimulus to cerebral cortex.Neurosurgery. 1987 Jan;20(1):74-93. Neurosurgery. 1987. PMID: 3543727 Review.
Cited by
-
Mouse corticospinal system comprises different functional neuronal ensembles depending on their hodology.BMC Neurosci. 2019 Sep 23;20(1):50. doi: 10.1186/s12868-019-0533-5. BMC Neurosci. 2019. PMID: 31547806 Free PMC article.
-
Post-stroke kinematic analysis in rats reveals similar reaching abnormalities as humans.Sci Rep. 2018 Jun 7;8(1):8738. doi: 10.1038/s41598-018-27101-0. Sci Rep. 2018. PMID: 29880827 Free PMC article.
-
Optimization of adeno-associated viral vector-mediated transduction of the corticospinal tract: comparison of four promoters.Gene Ther. 2021 Feb;28(1-2):56-74. doi: 10.1038/s41434-020-0169-1. Epub 2020 Jun 23. Gene Ther. 2021. PMID: 32576975 Free PMC article.
-
Recent advances in our understanding of the primate corticospinal system.F1000Res. 2019 Mar 11;8:F1000 Faculty Rev-274. doi: 10.12688/f1000research.17445.1. eCollection 2019. F1000Res. 2019. PMID: 30906528 Free PMC article. Review.
-
Five Breakthroughs: A First Approximation of Brain Evolution From Early Bilaterians to Humans.Front Neuroanat. 2021 Aug 17;15:693346. doi: 10.3389/fnana.2021.693346. eCollection 2021. Front Neuroanat. 2021. PMID: 34489649 Free PMC article.
References
-
- Alstermark B, Ogawa J & Isa T (2004). Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol 91, 1832–1839. - PubMed
-
- Altman J & Bayer SA (1980. a). Development of the brain stem in the rat. I. Thymidine‐radiographic study of the time of origin of neurons of the lower medulla. J Comp Neurol 194, 1–35. - PubMed
-
- Altman J & Bayer SA (1980. b). Development of the brain stem in the rat. II. Thymidine‐radiographic study of the time of origin of neurons of the upper medulla, excluding the vestibular and auditory nuclei. J Comp Neurol 194, 37–56. - PubMed
-
- Altman J & Bayer SA (1980. c). Development of the brain stem in the rat. III. Thymidine‐radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol 194, 877–904. - PubMed
-
- Altman J & Bayer SA (1980. d). Development of the brain stem in the rat. IV. Thymidine‐radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol 194, 905–929. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

