Moderate Sustained Virologic Response Rates With 6-Week Combination Directly Acting Anti-Hepatitis C Virus Therapy in Patients With Advanced Liver Disease
- PMID: 26503379
- PMCID: PMC4725378
- DOI: 10.1093/cid/civ897
Moderate Sustained Virologic Response Rates With 6-Week Combination Directly Acting Anti-Hepatitis C Virus Therapy in Patients With Advanced Liver Disease
Abstract
Background: Treatment of genotype 1 hepatitis C virus (HCV) infection with combination directly acting antivirals (DAA) for 8-24 weeks is associated with high rates of sustained virologic response (SVR). We previously demonstrated that adding a third DAA to ledipasvir and sofosbuvir (LDV/SOF) can result in high SVR rates in patients without cirrhosis. In this study, we investigated whether a similar regimen would yield equivalent rates of cure in patients with advanced liver fibrosis.
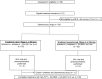
Methods: Fifty patients were enrolled at the Clinical Research Center of the National Institutes of Health and associated healthcare centers. Enrollment and follow-up data from April 2014 to June 2015 are reported here. Eligible participants were aged ≥18 years, had chronic HCV genotype 1 infection (serum HCV RNA ≥2000 IU/mL), and stage 3-4 liver fibrosis. HCV RNA was measured using a reverse-transcription polymerase chain reaction assay.
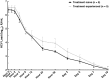
Results: Of patients treated with LDV, SOF, and the NS3/4A protease inhibitor GS-9451 for 6 weeks, 76% (38 of 50; 95% confidence interval, 60%-85%) had SVR achieved 12 weeks after the end of treatment. There was no statistically significant difference in treatment efficacy between treatment-naive patients (72%, 18 of 25) and those with treatment experience (80%; 20 of 25) (P = .51). Overall, 11 patients (22%) experienced virologic relapse, and 1 (2%) was lost to follow-up at 4 weeks after treatment. No serious adverse events, discontinuations, or deaths were associated with this regimen.
Conclusions: Adding a third DAA to LDV/SOF may result in a moderate SVR rate, lower than that observed in patients without cirrhosis. Significant liver fibrosis remains an impediment to achieving SVR with short-duration DAA therapy.
Chinese clinical trials registration: CT01805882.
Keywords: advanced fibrosis; hepatitis C; short-duration.
Published by Oxford University Press for the Infectious Diseases Society of America 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Fried MW, Shiffman ML, Reddy KR et al. . Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New Engl J Med 2002; 347:975–82. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC et al. . Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358:958–65. - PubMed
-
- Jacobson IM, McHutchison JG, Dusheiko G et al. . Telaprevir for previously untreated chronic hepatitis C virus infection. New Engl J Med 2011; 364:2405–16. - PubMed
-
- Kwo PY, Lawitz EJ, McCone J et al. . Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet 2010; 376:705–16. - PubMed
-
- Limaye AR, Draganov PV, Cabrera R. Boceprevir for chronic HCV genotype 1 infection. New Engl J Med 2011; 365:176–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

