The HRASLS (PLA/AT) subfamily of enzymes
- PMID: 26503625
- PMCID: PMC4624172
- DOI: 10.1186/s12929-015-0210-7
The HRASLS (PLA/AT) subfamily of enzymes
Abstract
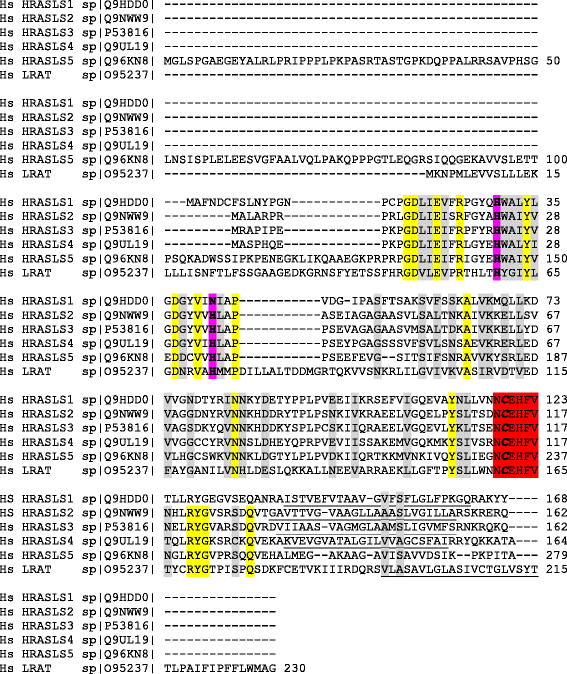
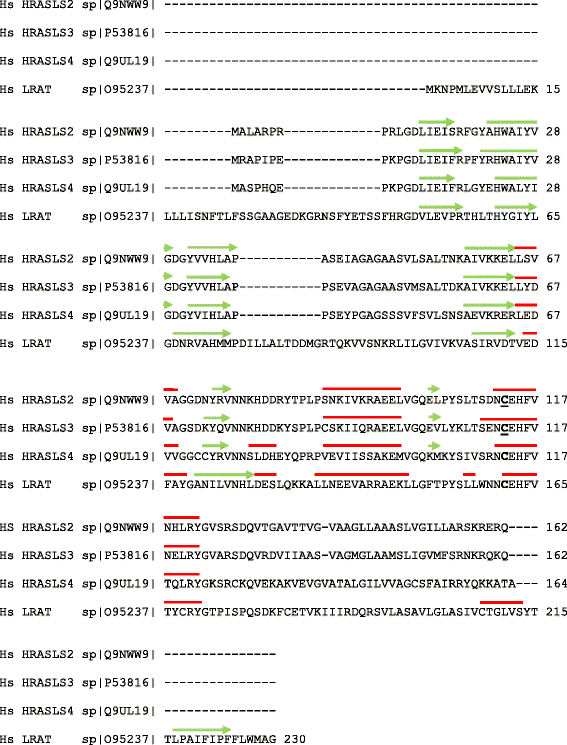
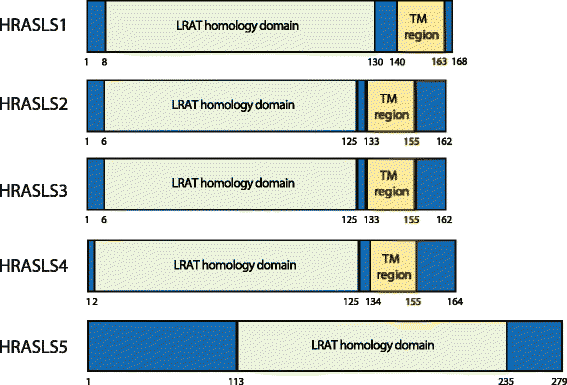
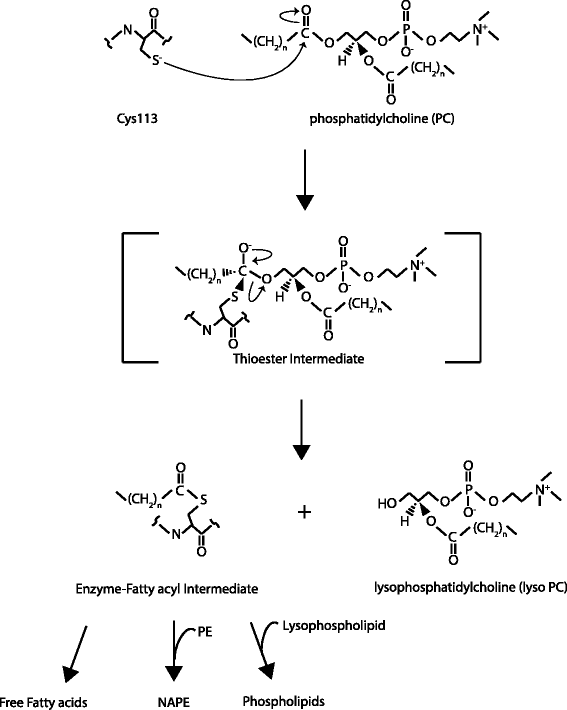
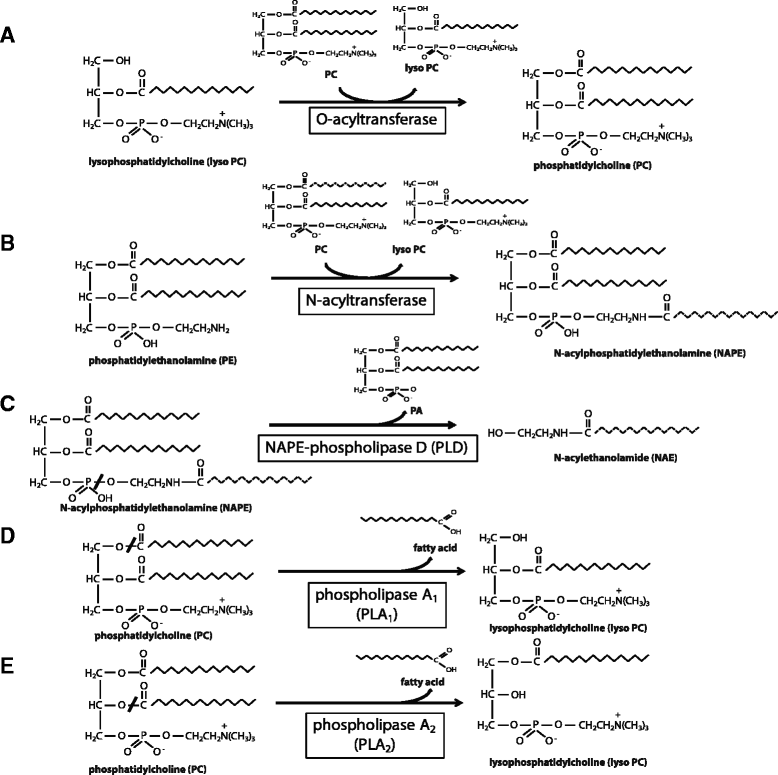
The H-RAS-like suppressor (HRASLS) subfamily consists of five enzymes (1-5) in humans and three (1, 3, and 5) in mice and rats that share sequence homology with lecithin:retinol acyltransferase (LRAT). All HRASLS family members possess in vitro phospholipid metabolizing abilities including phospholipase A1/2 (PLA1/2) activities and O-acyltransferase activities for the remodeling of glycerophospholipid acyl chains, as well as N-acyltransferase activities for the production of N-acylphosphatidylethanolamines. The in vivo biological activities of the HRASLS enzymes have not yet been fully investigated. Research to date indicates involvement of this subfamily in a wide array of biological processes and, as a consequence, these five enzymes have undergone extensive rediscovery and renaming within different fields of research. This review briefly describes the discovery of each of the HRASLS enzymes and their role in cancer, and discusses the biochemical function of each enzyme, as well as the biological role, if known. Gaps in current understanding are highlighted and suggestions for future research directions are discussed.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

