Modal affinities of endplate acetylcholine receptors caused by loop C mutations
- PMID: 26503719
- PMCID: PMC4621750
- DOI: 10.1085/jgp.201511503
Modal affinities of endplate acetylcholine receptors caused by loop C mutations
Abstract
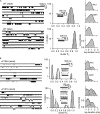
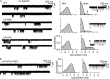
The time course of the endplate current is determined by the rate and equilibrium constants for acetylcholine receptor (AChR) activation. We measured these constants in single-channel currents from AChRs with mutations at the neurotransmitter-binding sites, in loop C. The main findings are: (a) Almost all perturbations of loop C generate heterogeneity in the channel open probability ("modes"). (b) Modes are generated by different affinities for ACh that can be either higher or lower than in the wild-type receptors. (c) The modes are stable, in so far as each receptor maintains its affinity for at least several minutes. (d) Different agonists show different degrees of modal activity. With the loop C mutation αP197A, there are four modes with ACh but only two with partial agonists. (e) The affinity variations arise exclusively from the αδ-binding site. (f) Substituting four γ-subunit residues into the δ subunit (three in loop E and one in the β5-β5' linker) reduces modal activity. (g) At each neurotransmitter-binding site, affinity is determined by a core of five aromatic residues. Modes are eliminated by an alanine mutation at δW57 but not at the other aromatics. (h) Modes are eliminated by a phenylalanine substitution at all core aromatics except αY93. The results suggest that, at the αδ agonist site, loop C and the complementary subunit surface can each adopt alternative conformations and interact with each other to influence the position of δW57 with respect to the aromatic core and, hence, affinity.
© 2015 Vij et al.
Figures







Comment in
-
Modal gating of endplate acetylcholine receptors: A proposed mechanism.J Gen Physiol. 2015 Dec;146(6):435-9. doi: 10.1085/jgp.201511534. J Gen Physiol. 2015. PMID: 26621772 Free PMC article. No abstract available.
References
-
- Beene D.L., Brandt G.S., Zhong W., Zacharias N.M., Lester H.A., and Dougherty D.A.. 2002. Cation-pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochemistry. 41:10262–10269. 10.1021/bi020266d - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

