The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial
- PMID: 26503762
- PMCID: PMC4995545
- DOI: 10.1038/mp.2015.162
The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial
Abstract
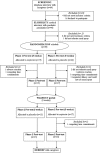
Interventions for autism are limited. The synthetic hormone oxytocin may provide a potential treatment to improve core social and behavioral difficulties in autism, but its efficacy has yet to be evaluated in young children who potentially may benefit to a greater extent. We investigated the efficacy, tolerability and safety of oxytocin treatment in young children with autism using a double-blind, randomized, placebo-controlled, crossover, clinical trial. Thirty-one children with autism received 12 International Units (IU) of oxytocin and placebo nasal spray morning and night (24 IU per day) for 5 weeks, with a 4-week washout period between each treatment. Compared with placebo, oxytocin led to significant improvements on the primary outcome of caregiver-rated social responsiveness. Overall, nasal spray was well tolerated, and the most common reported adverse events were thirst, urination and constipation. This study is the first clinical trial to support the potential of oxytocin as an early intervention for young children with autism to help improve social interaction deficits.
Figures


References
-
- Association APDiagnostic and Statistical Manual of Mental Disorders, 4th edn. Text Revision. American Psychiatric Association: Washington DC, USA, 2000.
-
- Autism Spectrum Disorders. Available at: http://www.cdc.gov/autism/ (last accessed date 20th November 2014).
-
- Sharma A, Shaw SR. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: a meta-analysis. J Pediatr Health Care 2012; 26: 291–299. - PubMed
-
- Gibbs TT Pharmacological treatment of autism. In: GJ Blatt (ed). The Neurochemical Basis of Autism. Springer: : Boston, MA, USA, 2010, pp 245–267.
-
- Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev 2012; 10: CD009260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

