Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors
- PMID: 26503962
- PMCID: PMC4744527
- DOI: 10.1158/2159-8290.CD-15-0583
Mesothelin-Targeted CARs: Driving T Cells to Solid Tumors
Abstract
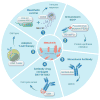
Chimeric antigen receptors (CAR) are synthetic receptors that target T cells to cell-surface antigens and augment T-cell function and persistence. Mesothelin is a cell-surface antigen implicated in tumor invasion, which is highly expressed in mesothelioma and lung, pancreas, breast, ovarian, and other cancers. Its low-level expression in mesothelia, however, commands thoughtful therapeutic interventions. Encouragingly, recent clinical trials evaluating active immunization or immunoconjugates in patients with pancreatic adenocarcinoma or mesothelioma have shown responses without toxicity. Altogether, these findings and preclinical CAR therapy models using either systemic or regional T-cell delivery argue favorably for mesothelin CAR therapy in multiple solid tumors.
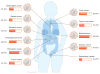
Significance: Recent success obtained with adoptive transfer of CAR T cells targeting CD19 in patients with refractory hematologic malignancies has generated much enthusiasm for T-cell engineering and raises the prospect of implementing similar strategies for solid tumors. Mesothelin is expressed in a wide range and a high percentage of solid tumors, which we review here in detail. Mesothelin CAR therapy has the potential to treat multiple solid malignancies.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–7. - PubMed
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

