HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction
- PMID: 26503986
- PMCID: PMC4692290
- DOI: 10.1093/cvr/cvv243
HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction
Erratum in
-
Corrigendum to: HIF-2α-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction.Cardiovasc Res. 2017 Feb;113(2):247. doi: 10.1093/cvr/cvw251. Epub 2017 Jan 12. Cardiovasc Res. 2017. PMID: 28087645 Free PMC article. No abstract available.
Abstract
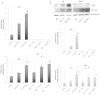
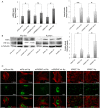
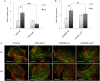
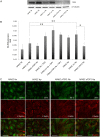
Aims: Hypoxic conditions stimulate pulmonary vasoconstriction and vascular remodelling, both pathognomonic changes in pulmonary arterial hypertension (PAH). The secreted protein thrombospondin-1 (TSP1) is involved in the maintenance of lung homeostasis. New work identified a role for TSP1 in promoting PAH. Nonetheless, it is largely unknown how hypoxia regulates TSP1 in the lung and whether this contributes to pathological events during PAH.
Methods and results: In cell and animal experiments, we found that hypoxia induces TSP1 in lungs, pulmonary artery smooth muscle cells and endothelial cells, and pulmonary fibroblasts. Using a murine model of constitutive hypoxia, gene silencing, and luciferase reporter experiments, we found that hypoxia-mediated induction of pulmonary TSP1 is a hypoxia-inducible factor (HIF)-2α-dependent process. Additionally, hypoxic tsp1(-/-) pulmonary fibroblasts and pulmonary artery smooth muscle cell displayed decreased migration compared with wild-type (WT) cells. Furthermore, hypoxia-mediated induction of TSP1 destabilized endothelial cell-cell interactions. This provides genetic evidence that TSP1 contributes to vascular remodelling during PAH. Expanding cell data to whole tissues, we found that, under hypoxia, pulmonary arteries (PAs) from WT mice had significantly decreased sensitivity to acetylcholine (Ach)-stimulated endothelial-dependent vasodilation. In contrast, hypoxic tsp1(-/-) PAs retained sensitivity to Ach, mediated in part by TSP1 regulation of pulmonary Kv channels. Translating these preclinical studies, we find in the lungs from individuals with end-stage PAH, both TSP1 and HIF-2α protein expression increased in the pulmonary vasculature compared with non-PAH controls.
Conclusions: These findings demonstrate that HIF-2α is clearly implicated in the TSP1 pulmonary regulation and provide new insights on its contribution to PAH-driven vascular remodelling and vasoconstriction.
Keywords: Endothelial cells; Fibroblasts; HIF-2α; Hypoxia; Pulmonary arterial hypertension; Pulmonary artery; Smooth muscle cells; Thrombospondin-1.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



Comment in
-
'Hypoxio-spondin': thrombospondin and its emerging role in pulmonary hypertension.Cardiovasc Res. 2016 Jan 1;109(1):1-3. doi: 10.1093/cvr/cvv258. Epub 2015 Nov 24. Cardiovasc Res. 2016. PMID: 26604038 No abstract available.
References
-
- Grunig E, Dehnert C, Mereles D, Koehler R, Olschewski H, Bartsch P, Janssen B. Enhanced hypoxic pulmonary vasoconstriction in families of adults or children with idiopathic pulmonary arterial hypertension. Chest 2005;128:630S–633S. - PubMed
-
- Lakshminrusimha S, Wiseman D, Black SM, Russell JA, Gugino SF, Oishi P, Steinhorn RH, Fineman JR. The role of nitric oxide synthase-derived reactive oxygen species in the altered relaxation of pulmonary arteries from lambs with increased pulmonary blood flow. Am J Physiol Heart Circ Physiol 2007;293:H1491–H1497. - PMC - PubMed
-
- Best DH, Austin ED, Chung WK, Elliott CG. Genetics of pulmonary hypertension. Curr Opin Cardiol 2014;29:520–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

