Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown
- PMID: 26504080
- PMCID: PMC4732197
- DOI: 10.1074/jbc.M115.696419
Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown
Abstract
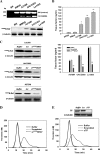
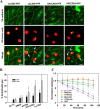
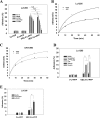
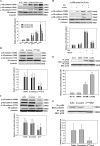
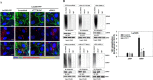
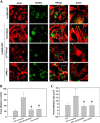
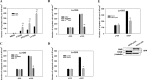
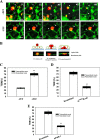
Tumor invasiveness depends on the ability of tumor cells to breach endothelial barriers. In this study, we investigated the mechanism by which the adhesion of melanoma cells to endothelium regulates adherens junction integrity and modulates tumor transendothelial migration (TEM) by initiating thrombin generation. We found that the B-Raf(V600E) mutation in metastatic melanoma cells up-regulated tissue factor (TF) expression on cell membranes and promoted thrombin production. Co-culture of endothelial monolayers with metastatic melanoma cells mediated the opening of inter-endothelial spaces near melanoma cell contact sites in the presence of platelet-free plasma (PFP). By using small interfering RNA (siRNA), we demonstrated that B-Raf(V600E) and TF silencing attenuated the focal disassembly of adherens junction induced by tumor contact. Vascular endothelial-cadherin (VE-cadherin) disassembly was dependent on phosphorylation of p120-catenin on Ser-879 and VE-cadherin on Tyr-658, Tyr-685, and Tyr-731, which can be prevented by treatment with the thrombin inhibitor, hirudin, or by silencing the thrombin receptor, protease-activated receptor-1, in endothelial cells. We also provided strong evidence that tumor-derived thrombin enhanced melanoma TEM by inducing ubiquitination-coupled VE-cadherin internalization, focal adhesion formation, and actin assembly in endothelium. Confocal microscopic analysis of tumor TEM revealed that junctions transiently opened and resealed as tumor cells accomplished TEM. In addition, in the presence of PFP, tumor cells preferentially transmigrated via paracellular routes. PFP supported melanoma transmigration under shear conditions via a B-Raf(V600E)-thrombin-dependent mechanism. We concluded that the activation of thrombin generation by cancer cells in plasma is an important process regulating melanoma extravasation by disrupting endothelial junction integrity.
Keywords: V600EB-raf; VE-cadherin; adherens junction; focal adhesion; thrombin; tissue factor; tumor metastasis.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
CD44 variant, but not standard CD44 isoforms, mediate disassembly of endothelial VE-cadherin junction on metastatic melanoma cells.FEBS Lett. 2014 Dec 20;588(24):4573-82. doi: 10.1016/j.febslet.2014.10.027. Epub 2014 Nov 4. FEBS Lett. 2014. PMID: 25447529
-
p38 MAP kinase is necessary for melanoma-mediated regulation of VE-cadherin disassembly.Am J Physiol Cell Physiol. 2010 May;298(5):C1140-50. doi: 10.1152/ajpcell.00242.2009. Epub 2010 Feb 24. Am J Physiol Cell Physiol. 2010. PMID: 20181932 Free PMC article.
-
ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.J Immunol. 2007 Sep 15;179(6):4053-64. doi: 10.4049/jimmunol.179.6.4053. J Immunol. 2007. PMID: 17785844
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
Cell-cell interactions during transendothelial migration of tumor cells.Microsc Res Tech. 1998 Nov 1;43(3):265-75. doi: 10.1002/(SICI)1097-0029(19981101)43:3<265::AID-JEMT9>3.0.CO;2-Z. Microsc Res Tech. 1998. PMID: 9840805 Review.
Cited by
-
Activating Sphingosine-1-phospahte signaling in endothelial cells increases myosin light chain phosphorylation to decrease endothelial permeability thereby inhibiting cancer metastasis.Cancer Lett. 2021 May 28;506:107-119. doi: 10.1016/j.canlet.2021.01.004. Epub 2021 Feb 16. Cancer Lett. 2021. PMID: 33600895 Free PMC article.
-
Junctional proteins of the blood-brain barrier: New insights into function and dysfunction.Tissue Barriers. 2016 Feb 26;4(1):e1154641. doi: 10.1080/21688370.2016.1154641. eCollection 2016 Jan-Mar. Tissue Barriers. 2016. PMID: 27141427 Free PMC article. Review.
-
Rho, ROCK and actomyosin contractility in metastasis as drug targets.F1000Res. 2016 Apr 29;5:F1000 Faculty Rev-783. doi: 10.12688/f1000research.7909.1. eCollection 2016. F1000Res. 2016. PMID: 27158478 Free PMC article. Review.
-
Platelet-Based Nanoparticles with Stimuli-Responsive for Anti-Tumor Therapy.Int J Nanomedicine. 2023 Nov 6;18:6293-6309. doi: 10.2147/IJN.S436373. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37954456 Free PMC article. Review.
-
Cortical Actin Dynamics in Endothelial Permeability.Curr Top Membr. 2018;82:141-195. doi: 10.1016/bs.ctm.2018.09.003. Epub 2018 Oct 15. Curr Top Membr. 2018. PMID: 30360779 Free PMC article.
References
-
- Konstantopoulos K., and Thomas S. N. (2009) Cancer cells in transit: the vascular interactions of tumor cells. Annu. Rev. Biomed. Eng. 11, 177–202 - PubMed
-
- Biggerstaff J. P., Seth N., Amirkhosravi A., Amaya M., Fogarty S., Meyer T. V., Siddiqui F., and Francis J. L. (1999) Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastasis. Clin. Exp. Metastasis 17, 723–730 - PubMed
-
- Ruf W., and Edgington T. S. (1994) Structural biology of tissue factor, the initiator of thrombogenesis in vivo. FASEB J. 8, 385–390 - PubMed
-
- Brummel K. E., Paradis S. G., Butenas S., and Mann K. G. (2002) Thrombin functions during tissue factor-induced blood coagulation. Blood 100, 148–152 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

