Retinal venous pressure: the role of endothelin
- PMID: 26504500
- PMCID: PMC4620652
- DOI: 10.1186/s13167-015-0043-1
Retinal venous pressure: the role of endothelin
Abstract
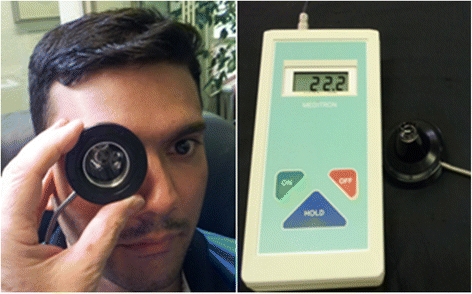
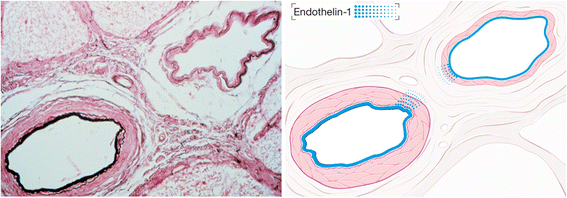
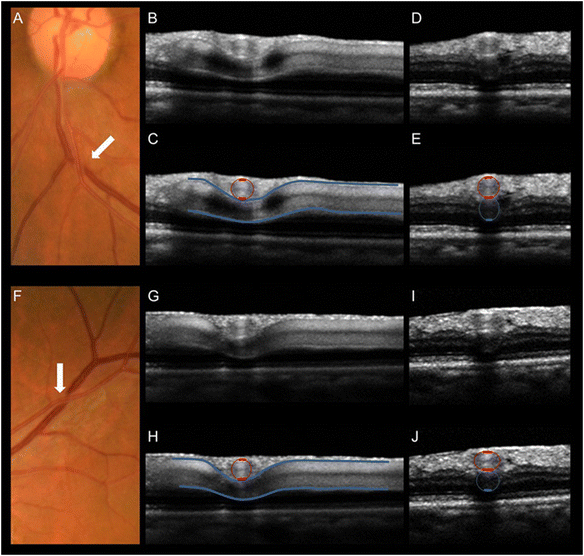
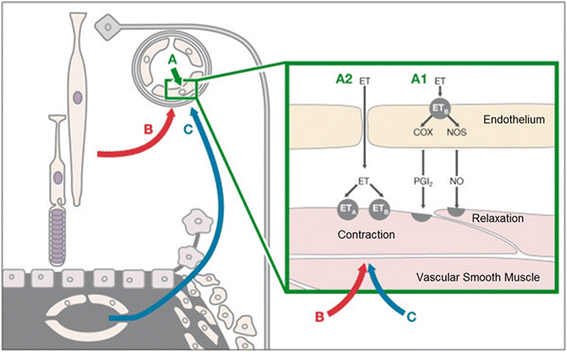
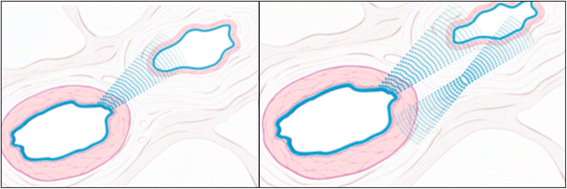
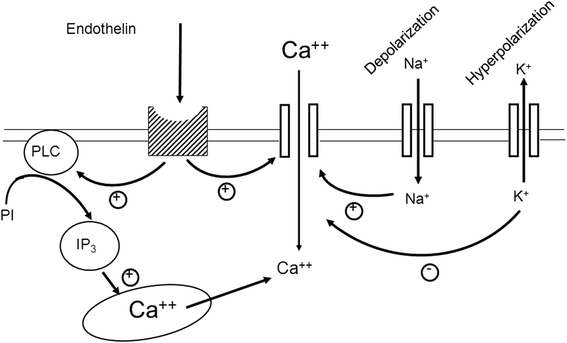
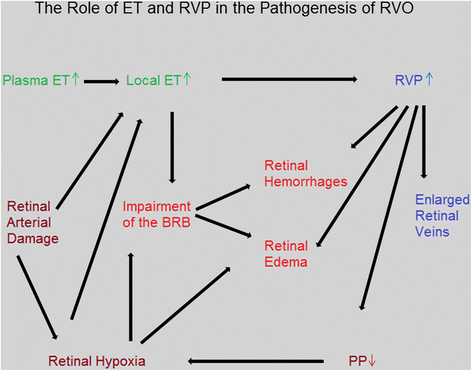
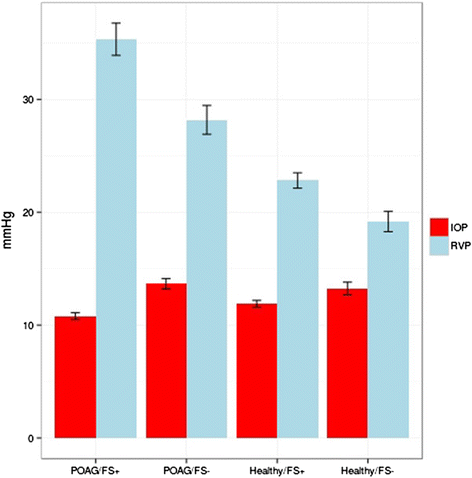
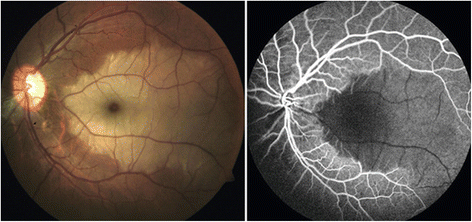
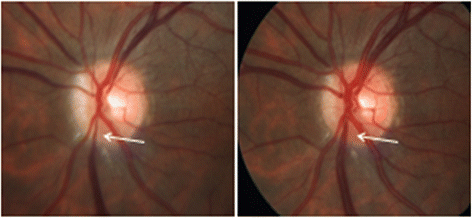
The retinal venous pressure (RVP) can be measured non-invasively. While RVP is equal to or slightly above intraocular pressure (IOP) in healthy people, it is often markedly increased in patients with eye or systemic diseases. Beside a mechanical obstruction, the main cause of such an elevation is a local dysregulation of a retinal vein, particularly a constriction induced by endothelin-1 (ET-1). A local increase of ET-1 can result from a high plasma level, as ET-1 can diffuse from the fenestrated capillaries of the choroid into the optic nerve head (ONH), bypassing the blood retinal barrier. A local increase can also result from increased local production either by a sick neighboring artery or retinal tissue. Generally, the main factors increasing ET-1 are inflammations and hypoxia, either locally or in a remote organ. RVP is known to be increased in patients with glaucoma, retinal vein occlusion (RVO), diabetic retinopathy, high mountain disease, and primary vascular dysregulation (PVD). PVD is the major vascular component of Flammer syndrome (FS). An increase of RVP decreases perfusion pressure, which heightens the risk for hypoxia. An increase of RVP also elevates transmural pressure, which in turn heightens the risk for retinal edema. In patients with RVO, a high level of RVP may not only be a consequence but also a potential cause of the occlusion; therefore, it risks causing a vicious circle. Narrow retinal arteries and particularly dilated retinal veins are known risk indicators for future cardiovascular events. As the major cause for such a retinal venous dilatation is an increased RVP, RVP may likely turn out to be an even stronger predictor.
Keywords: Diabetes mellitus; Dilated retinal veins; Endothelin-1 (ET-1); Flammer syndrome (FS); Glaucoma; Ophthalmodynamometry; Predictive, preventive and personalized medicine; Primary vascular dysregulation (PVD); Retinal vein occlusion (RVO); Retinal venous pressure (RVP); Venous constriction.
Figures





References
-
- Baertschi M. Factors influencing retinal venous pressure. Doctoral thesis SALUS University; 2015.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

