Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model
- PMID: 26505753
- PMCID: PMC4624431
- DOI: 10.1371/journal.pmed.1001891
Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model
Abstract
Background: The undetectable hypnozoite reservoir for relapsing Plasmodium vivax and P. ovale malarias presents a major challenge for malaria control and elimination in endemic countries. This study aims to directly determine the contribution of relapses to the burden of P. vivax and P. ovale infection, illness, and transmission in Papua New Guinean children.
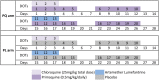
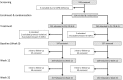
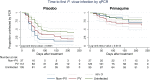
Methods and findings: From 17 August 2009 to 20 May 2010, 524 children aged 5-10 y from East Sepik Province in Papua New Guinea (PNG) participated in a randomised double-blind placebo-controlled trial of blood- plus liver-stage drugs (chloroquine [CQ], 3 d; artemether-lumefantrine [AL], 3 d; and primaquine [PQ], 20 d, 10 mg/kg total dose) (261 children) or blood-stage drugs only (CQ, 3 d; AL, 3 d; and placebo [PL], 20 d) (263 children). Participants, study staff, and investigators were blinded to the treatment allocation. Twenty children were excluded during the treatment phase (PQ arm: 14, PL arm: 6), and 504 were followed actively for 9 mo. During the follow-up time, 18 children (PQ arm: 7, PL arm: 11) were lost to follow-up. Main primary and secondary outcome measures were time to first P. vivax infection (by qPCR), time to first clinical episode, force of infection, gametocyte positivity, and time to first P. ovale infection (by PCR). A basic stochastic transmission model was developed to estimate the potential effect of mass drug administration (MDA) for the prevention of recurrent P. vivax infections. Targeting hypnozoites through PQ treatment reduced the risk of having at least one qPCR-detectable P. vivax or P. ovale infection during 8 mo of follow-up (P. vivax: PQ arm 0.63/y versus PL arm 2.62/y, HR = 0.18 [95% CI 0.14, 0.25], p < 0.001; P. ovale: 0.06 versus 0.14, HR = 0.31 [95% CI 0.13, 0.77], p = 0.011) and the risk of having at least one clinical P. vivax episode (HR = 0.25 [95% CI 0.11, 0.61], p = 0.002). PQ also reduced the molecular force of P. vivax blood-stage infection in the first 3 mo of follow-up (PQ arm 1.90/y versus PL arm 7.75/y, incidence rate ratio [IRR] = 0.21 [95% CI 0.15, 0.28], p < 0.001). Children who received PQ were less likely to carry P. vivax gametocytes (IRR = 0.27 [95% CI 0.19, 0.38], p < 0.001). PQ had a comparable effect irrespective of the presence of P. vivax blood-stage infection at the time of treatment (p = 0.14). Modelling revealed that mass screening and treatment with highly sensitive quantitative real-time PCR, or MDA with blood-stage treatment alone, would have only a transient effect on P. vivax transmission levels, while MDA that includes liver-stage treatment is predicted to be a highly effective strategy for P. vivax elimination. The inclusion of a directly observed 20-d treatment regime maximises the efficiency of hypnozoite clearance but limits the generalisability of results to real-world MDA programmes.
Conclusions: These results suggest that relapses cause approximately four of every five P. vivax infections and at least three of every five P. ovale infections in PNG children and are important in sustaining transmission. MDA campaigns combining blood- and liver-stage treatment are predicted to be a highly efficacious intervention for reducing P. vivax and P. ovale transmission.
Trial registration: ClinicalTrials.gov NCT02143934.
Conflict of interest statement
The authors have declared the following competing interests: IM has acted as an Academic Editor for
Figures



References
-
- World Health Organization. World malaria report 2014. Geneva: World Health Organization; 2014.
-
- Pan American Health Organization, World Health Organization. Central America and Hispaniola seek to eliminate malaria by 2025. 21 May 2013. Geneva: World Health Organization.
-
- Asia Pacific Leaders Malaria Alliance. East Asia Summit adopts unprecedented regional malaria goal. 14 November 2014. Mandaluyong City (Philippines): Asia Pacific Leaders Malaria Alliance.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

