Coordination of planar cell polarity pathways through Spiny-legs
- PMID: 26505959
- PMCID: PMC4764577
- DOI: 10.7554/eLife.09946
Coordination of planar cell polarity pathways through Spiny-legs
Abstract
Morphogenesis and physiology of tissues and organs requires planar cell polarity (PCP) systems that orient and coordinate cells and their behaviors, but the relationship between PCP systems has been controversial. We have characterized how the Frizzled and Dachsous-Fat PCP systems are connected through the Spiny-legs isoform of the Prickle-Spiny-legs locus. Two different components of the Dachsous-Fat system, Dachsous and Dachs, can each independently interact with Spiny-legs and direct its localization in vivo. Through characterization of the contributions of Prickle, Spiny-legs, Dachsous, Fat, and Dachs to PCP in the Drosophila wing, eye, and abdomen, we define where Dachs-Spiny-legs and Dachsous-Spiny-legs interactions contribute to PCP, and provide a new understanding of the orientation of polarity and the basis of PCP phenotypes. Our results support the direct linkage of PCP systems through Sple in specific locales, while emphasizing that cells can be subject to and must ultimately resolve distinct, competing PCP signals.
Keywords: D. melanogaster; cell biology; developmental biology; fat; pcp; polarity; prickle; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


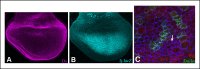
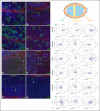
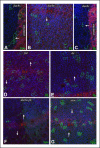


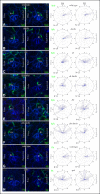


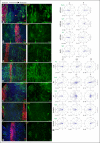

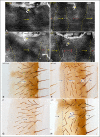
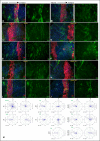
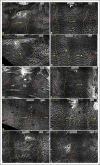

References
-
- Ayukawa T, Akiyama M, Mummery-Widmer Jennifer L, Stoeger T, Sasaki J, Knoblich Juergen A, Senoo H, Sasaki T, Yamazaki M. Dachsous-dependent asymmetric localization of spiny-legs determines planar cell polarity orientation in drosophila. Cell Reports. 2014;8:610–621. doi: 10.1016/j.celrep.2014.06.009. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

