Combinations of PARP Inhibitors with Temozolomide Drive PARP1 Trapping and Apoptosis in Ewing's Sarcoma
- PMID: 26505995
- PMCID: PMC4624427
- DOI: 10.1371/journal.pone.0140988
Combinations of PARP Inhibitors with Temozolomide Drive PARP1 Trapping and Apoptosis in Ewing's Sarcoma
Abstract
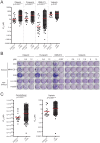
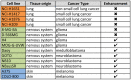
Ewing's sarcoma is a malignant pediatric bone tumor with a poor prognosis for patients with metastatic or recurrent disease. Ewing's sarcoma cells are acutely hypersensitive to poly (ADP-ribose) polymerase (PARP) inhibition and this is being evaluated in clinical trials, although the mechanism of hypersensitivity has not been directly addressed. PARP inhibitors have efficacy in tumors with BRCA1/2 mutations, which confer deficiency in DNA double-strand break (DSB) repair by homologous recombination (HR). This drives dependence on PARP1/2 due to their function in DNA single-strand break (SSB) repair. PARP inhibitors are also cytotoxic through inhibiting PARP1/2 auto-PARylation, blocking PARP1/2 release from substrate DNA. Here, we show that PARP inhibitor sensitivity in Ewing's sarcoma cells is not through an apparent defect in DNA repair by HR, but through hypersensitivity to trapped PARP1-DNA complexes. This drives accumulation of DNA damage during replication, ultimately leading to apoptosis. We also show that the activity of PARP inhibitors is potentiated by temozolomide in Ewing's sarcoma cells and is associated with enhanced trapping of PARP1-DNA complexes. Furthermore, through mining of large-scale drug sensitivity datasets, we identify a subset of glioma, neuroblastoma and melanoma cell lines as hypersensitive to the combination of temozolomide and PARP inhibition, potentially identifying new avenues for therapeutic intervention. These data provide insights into the anti-cancer activity of PARP inhibitors with implications for the design of treatment for Ewing's sarcoma patients with PARP inhibitors.
Conflict of interest statement
Figures




References
-
- Macmillan Cancer Support. Ewing's sarcoma of the bone 2014 [updated March 1, 2014]; Available: http://www.macmillan.org.uk/Cancerinformation/Cancertypes/Bone/Typesofbo.... Accessed 30 September 2014.
-
- National Cancer Institute at the National Institutes of Health. Ewing Sarcoma Treatment: Treatment Option Overview 2013 [updated October 18, 2013]. Available: http://www.cancer.gov/cancertopics/pdq/treatment/ewings/HealthProfession....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

