Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug
- PMID: 26506265
- PMCID: PMC4639806
- DOI: 10.1038/ncomms9466
Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug
Abstract
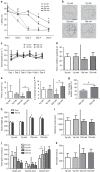
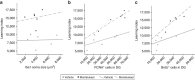
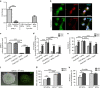
As human life expectancy has improved rapidly in industrialized societies, age-related cognitive impairment presents an increasing challenge. Targeting histopathological processes that correlate with age-related cognitive declines, such as neuroinflammation, low levels of neurogenesis, disrupted blood-brain barrier and altered neuronal activity, might lead to structural and functional rejuvenation of the aged brain. Here we show that a 6-week treatment of young (4 months) and old (20 months) rats with montelukast, a marketed anti-asthmatic drug antagonizing leukotriene receptors, reduces neuroinflammation, elevates hippocampal neurogenesis and improves learning and memory in old animals. By using gene knockdown and knockout approaches, we demonstrate that the effect is mediated through inhibition of the GPR17 receptor. This work illustrates that inhibition of leukotriene receptor signalling might represent a safe and druggable target to restore cognitive functions in old individuals and paves the way for future clinical translation of leukotriene receptor inhibition for the treatment of dementias.
Conflict of interest statement
L.A., S.C.-D. and J.M. are inventors on the patent application WO 2014090990 A1 ‘Leukotriene pathway antagonists for the treatment of dementia, cognitive deficits in parkinson's disease and/or learning and memory deficiencies in parkinson's disease'. All other authors declare no competing financial interests.
Figures




Similar articles
-
Pharmaceutical Rejuvenation of Age-Associated Decline in Spatial Memory.Rejuvenation Res. 2016 Dec;19(6):521-524. doi: 10.1089/rej.2016.1903. Rejuvenation Res. 2016. PMID: 27881050
-
Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice.Neuropharmacology. 2014 Apr;79:707-14. doi: 10.1016/j.neuropharm.2014.01.011. Epub 2014 Jan 20. Neuropharmacology. 2014. PMID: 24456746
-
Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats.Brain Res. 2013 Sep 12;1530:1-12. doi: 10.1016/j.brainres.2013.07.035. Epub 2013 Jul 24. Brain Res. 2013. PMID: 23891717
-
More is less: neurogenesis and age-related cognitive decline in Long-Evans rats.Sci Aging Knowledge Environ. 2005 Feb 16;2005(7):re2. doi: 10.1126/sageke.2005.7.re2. Sci Aging Knowledge Environ. 2005. PMID: 15716513 Review.
-
Blood-Borne Revitalization of the Aged Brain.JAMA Neurol. 2015 Oct;72(10):1191-4. doi: 10.1001/jamaneurol.2015.1616. JAMA Neurol. 2015. PMID: 26237737 Free PMC article. Review.
Cited by
-
Potential of Nanotechnology-based Formulations in Combating Pulmonary Infectious Diseases: A Current Scenario.Curr Pharm Des. 2022;28(42):3413-3427. doi: 10.2174/1381612829666221116143138. Curr Pharm Des. 2022. PMID: 36397631 Review.
-
Montelukast Nanocrystals for Transdermal Delivery with Improved Chemical Stability.Pharmaceutics. 2019 Dec 23;12(1):18. doi: 10.3390/pharmaceutics12010018. Pharmaceutics. 2019. PMID: 31877986 Free PMC article.
-
Leukotriene receptor antagonist use and cognitive decline in normal cognition, mild cognitive impairment, and Alzheimer's dementia.Alzheimers Res Ther. 2021 Sep 3;13(1):147. doi: 10.1186/s13195-021-00892-7. Alzheimers Res Ther. 2021. PMID: 34479635 Free PMC article.
-
Discovery of 3-Substituted 1H-Indole-2-carboxylic Acid Derivatives as a Novel Class of CysLT1 Selective Antagonists.ACS Med Chem Lett. 2016 Jan 22;7(3):335-9. doi: 10.1021/acsmedchemlett.5b00482. eCollection 2016 Mar 10. ACS Med Chem Lett. 2016. PMID: 26985325 Free PMC article.
-
Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases.Mediators Inflamm. 2017;2017:3454212. doi: 10.1155/2017/3454212. Epub 2017 May 10. Mediators Inflamm. 2017. PMID: 28607533 Free PMC article. Review.
References
-
- Sierra A., Gottfried-Blackmore A. C., McEwen B. S. & Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

