Randomized Clinical Trial of Family-Based Treatment and Cognitive-Behavioral Therapy for Adolescent Bulimia Nervosa
- PMID: 26506579
- PMCID: PMC4624104
- DOI: 10.1016/j.jaac.2015.08.008
Randomized Clinical Trial of Family-Based Treatment and Cognitive-Behavioral Therapy for Adolescent Bulimia Nervosa
Abstract
Objective: There is a paucity of randomized clinical trials (RCTs) for adolescents with bulimia nervosa (BN). Prior studies suggest cognitive-behavioral therapy adapted for adolescents (CBT-A) and family-based treatment for adolescent bulimia nervosa (FBT-BN) could be effective for this patient population. The objective of this study was to compare the relative efficacy of these 2 specific therapies, FBT-BN and CBT-A. In addition, a smaller participant group was randomized to a nonspecific treatment (supportive psychotherapy [SPT]), whose data were to be used if there were no differences between FBT-BN and CBT-A at end of treatment.
Method: This 2-site (Chicago and Stanford) randomized controlled trial included 130 participants (aged 12-18 years) meeting DSM-IV criteria for BN or partial BN (binge eating and purging once or more per week for 6 months). Outcomes were assessed at baseline, end of treatment, and 6 and 12 months posttreatment. Treatments involved 18 outpatient sessions over 6 months. The primary outcome was defined as abstinence from binge eating and purging for 4 weeks before assessment, using the Eating Disorder Examination.
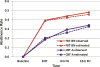
Results: Participants in FBT-BN achieved higher abstinence rates than in CBT-A at end of treatment (39% versus 20%; p = .040, number needed to treat [NNT] = 5) and at 6-month follow-up (44% versus 25%; p = .030, NNT = 5). Abstinence rates between these 2 groups did not differ statistically at 12-month follow-up (49% versus 32%; p = .130, NNT = 6).
Conclusion: In this study, FBT-BN was more effective in promoting abstinence from binge eating and purging than CBT-A in adolescent BN at end of treatment and 6-month follow-up. By 12-month follow-up, there were no statistically significant differences between the 2 treatments.
Clinical trial registration information: Study of Treatment for Adolescents With Bulimia Nervosa; http://clinicaltrials.gov/; NCT00879151.
Keywords: adolescent medicine; bulimia nervosa; cognitive-behavioral therapy; eating disorders; family-based treatment.
Copyright © 2015 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- van Hoeken D, Seidell J, Hoek HW. Epidemiology. In: Treasure J, Schmidt U, van Furth E, editors. Handbook of Eating Disorders. 2nd. Chinchester, England: John Wiley and Sons, Inc; 2004. pp. 11–34.
-
- Rome ES, Ammerman S. Medical complications of eating disorders: an update. J Adolesc Health. 2003;33(6):418–426. - PubMed
-
- Crow SJ, Swanson SA, Le Grange D, Feig EH, Merikangas KR. Suicidal behavior in adolescents and adults with bulimia nervosa. Compr Psychiatry. 2014;55(7):1534–9. - PubMed
-
- Godart NT, Flament MF, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: a review. Int J Eat Disord. 2002;32(3):253–270. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

