Inhibition of Human Hepatic Bile Acid Transporters by Tolvaptan and Metabolites: Contributing Factors to Drug-Induced Liver Injury?
- PMID: 26507107
- PMCID: PMC6169477
- DOI: 10.1093/toxsci/kfv231
Inhibition of Human Hepatic Bile Acid Transporters by Tolvaptan and Metabolites: Contributing Factors to Drug-Induced Liver Injury?
Abstract
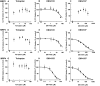
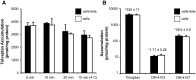
Tolvaptan is a vasopressin V(2)-receptor antagonist that has shown promise in treating Autosomal Dominant Polycystic Kidney Disease (ADPKD). Tolvaptan was, however, associated with liver injury in some ADPKD patients. Inhibition of bile acid transporters may be contributing factors to drug-induced liver injury. In this study, the ability of tolvaptan and two metabolites, DM-4103 and DM-4107, to inhibit human hepatic transporters (NTCP, BSEP, MRP2, MRP3, and MRP4) and bile acid transport in sandwich-cultured human hepatocytes (SCHH) was explored. IC(50) values were determined for tolvaptan, DM-4103 and DM-4107 inhibition of NTCP (∼41.5, 16.3, and 95.6 μM, respectively), BSEP (31.6, 4.15, and 119 μM, respectively), MRP2 (>50, ∼51.0, and >200 μM, respectively), MRP3 (>50, ∼44.6, and 61.2 μM, respectively), and MRP4 (>50, 4.26, and 37.9 μM, respectively). At the therapeutic dose of tolvaptan (90 mg), DM-4103 exhibited a C(max)/IC(50) value >0.1 for NTCP, BSEP, MRP2, MRP3, and MRP4. Tolvaptan accumulation in SCHH was extensive and not sodium-dependent; intracellular concentrations were ∼500 μM after a 10-min incubation duration with tolvaptan (15 μM). The biliary clearance of taurocholic acid (TCA) decreased by 43% when SCHH were co-incubated with tolvaptan (15 μM) and TCA (2.5 μM). When tolvaptan (15 μM) was co-incubated with 2.5 μM of chenodeoxycholic acid, taurochenodeoxycholic acid, or glycochenodeoxycholic acid in separate studies, the cellular accumulation of these bile acids increased by 1.30-, 1.68-, and 2.16-fold, respectively. Based on these data, inhibition of hepatic bile acid transport may be one of the biological mechanisms underlying tolvaptan-associated liver injury in patients with ADPKD.
Keywords: bile acids; drug-induced liver injury; hepatic transporters.
© The Author 2015. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Akaike H. (1974). A new look at the statistical model identification. IEEE Trans. Auto. Control AC-19, 716–723.
-
- Akita H., Suzuki H., Hirohashi T., Takikawa H., Sugiyama Y. (2002). Transport activity of human MRP3 expressed in Sf9 cells: comparative studies with rat MRP3. Pharm. Res. 19, 34–41. - PubMed
-
- Boertien W. E., Meijer E., de Jong P. E., ter Horst G. J., Renken R. J., van der Jagt E. J., Kappert P., Ouyang J., Engels G. E., van Oeveren W., et al. (2015). Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am. J. Kidney Dis. 65, 833–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

