Characterization of the Functional Roles of Amino Acid Residues in Acceptor-binding Subsite +1 in the Active Site of the Glucansucrase GTF180 from Lactobacillus reuteri 180
- PMID: 26507662
- PMCID: PMC4705985
- DOI: 10.1074/jbc.M115.687558
Characterization of the Functional Roles of Amino Acid Residues in Acceptor-binding Subsite +1 in the Active Site of the Glucansucrase GTF180 from Lactobacillus reuteri 180
Abstract
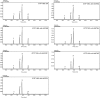
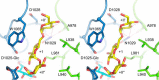
α-Glucans produced by glucansucrase enzymes hold strong potential for industrial applications. The exact determinants of the linkage specificity of glucansucrase enzymes have remained largely unknown, even with the recent elucidation of glucansucrase crystal structures. Guided by the crystal structure of glucansucrase GTF180-ΔN from Lactobacillus reuteri 180 in complex with the acceptor substrate maltose, we identified several residues (Asp-1028 and Asn-1029 from domain A, as well as Leu-938, Ala-978, and Leu-981 from domain B) near subsite +1 that may be critical for linkage specificity determination, and we investigated these by random site-directed mutagenesis. First, mutants of Ala-978 (to Leu, Pro, Phe, or Tyr) and Asp-1028 (to Tyr or Trp) with larger side chains showed reduced degrees of branching, likely due to the steric hindrance by these bulky residues. Second, Leu-938 mutants (except L938F) and Asp-1028 mutants showed altered linkage specificity, mostly with increased (α1 → 6) linkage synthesis. Third, mutation of Leu-981 and Asn-1029 significantly affected the transglycosylation reaction, indicating their essential roles in acceptor substrate binding. In conclusion, glucansucrase product specificity is determined by an interplay of domain A and B residues surrounding the acceptor substrate binding groove. Residues surrounding the +1 subsite thus are critical for activity and specificity of the GTF180 enzyme and play different roles in the enzyme functions. This study provides novel insights into the structure-function relationships of glucansucrase enzymes and clearly shows the potential of enzyme engineering to produce tailor-made α-glucans.
Keywords: GTF180; carbohydrate; enzyme mutation; glucansucrase; polysaccharide; product specificity; protein engineering; site-directed mutagenesis; α-glucan.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Badel S., Bernardi T., and Michaud P. (2011) New perspectives for lactobacilli exopolysaccharides. Biotechnol. Adv. 29, 54–66 - PubMed
-
- Jolly L., Vincent S. J., Duboc P., and Neeser J. R. (2002) Exploiting expolysaccharides from lactic acid bacteria. Antonie Van Leeuwenhoek 82, 367–374 - PubMed
-
- Monsan P., Bozonnet S., Albenne C., Joucla G., Willemot R.-M., and Remaud-Siméon M. (2001) Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 11, 675–685
-
- Welman A. D., and Maddox I. S. (2003) Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21, 269–274 - PubMed
-
- Leemhuis H., Pijning T., Dobruchowska J. M., van Leeuwen S. S., Kralj S., Dijkstra B. W., and Dijkhuizen L. (2013) Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 163, 250–272 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

