Sustained weight loss in patients treated with mifepristone for Cushing's syndrome: a follow-up analysis of the SEISMIC study and long-term extension
- PMID: 26507877
- PMCID: PMC4624667
- DOI: 10.1186/s12902-015-0059-5
Sustained weight loss in patients treated with mifepristone for Cushing's syndrome: a follow-up analysis of the SEISMIC study and long-term extension
Abstract
Background: Overweight and obesity are common among patients with Cushing's syndrome (CS) and may persist in some patients even after ostensibly curative surgery, contributing to cardiometabolic dysfunction and increased cardiovascular risk. Mifepristone, a selective glucocorticoid receptor antagonist, was effective in controlling hyperglycemia in a 24-week trial of adults (N = 50) with endogenous CS and associated type 2 diabetes mellitus/impaired glucose tolerance or hypertension who had failed or were not candidates for surgery (SEISMIC, Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing's Syndrome). This analysis examines long-term weight change among patients who received mifepristone in SEISMIC and enrolled in a long-term safety extension (LTE) study.
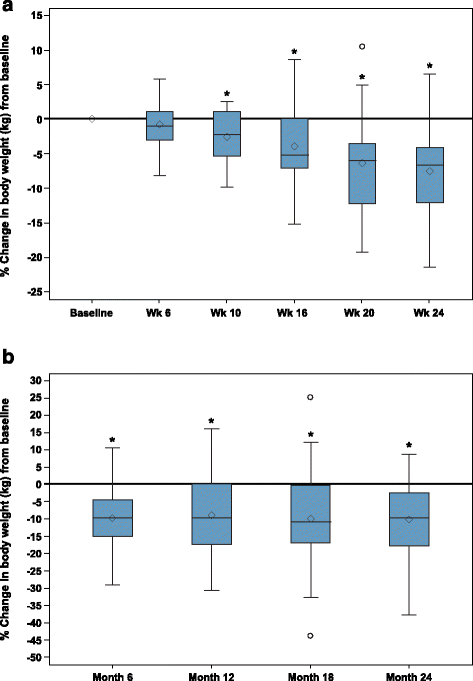
Methods: Patients completing the 24-week SEISMIC study and subsequent 6-week off-drug safety evaluation were invited to enroll in the LTE study. Mifepristone doses at the end of SEISMIC were the LTE starting doses. Body weight measures were reviewed at baseline and week 24 of SEISMIC and at LTE month 6, 12, 18, 24, and final visit (last observation collected during the LTE study).
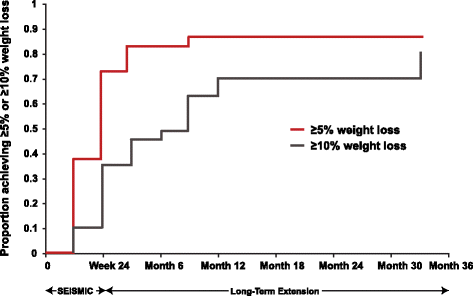
Results: Of the 30 patients enrolled in the LTE, evaluable weight data were available for 29 (20/29 female; mean age of 44.7 ± 11.2 years). These patients received mifepristone for a median of 29.2 months (range 8.4-41.9). Mean ± SD weight from SEISMIC baseline to LTE final visit decreased by 10.3 ± 16.3 kg (mean 105.4 ± 34.3 kg to 95.1 ± 32.9 kg), a 9.3 % decrease from baseline weight (P = 0.0008). Of the 29 LTE patients, 18 (62.1 %) lost ≥ 5 % of body weight by the end of the initial 24-week treatment period; this ≥5 % weight loss persisted in 83.3 % (15/18) at LTE final visit. Ten patients (34.5 %) lost ≥ 10 % of initial body weight by week 24 of SEISMIC, which persisted in 80 % at LTE final visit. No new safety signals were detected with long-term mifepristone use.
Conclusion: Clinically meaningful weight loss achieved during a 24-week study of mifepristone for CS persisted for two additional years in patients who remained on therapy. Long-term treatment with mifepristone appears to have a beneficial effect on weight in patients with endogenous CS.
Trial registration: NCT00569582 (SEISMIC); NCT00936741 (Long-Term Extension).
Figures

References
-
- Ntali G, Asimakopoulou A, Siamatras T, Komninos J, Vassiliadi D, Tzanela M, Tsagarakis S, Grossman AB, Wass JAH, Karavitaki N. Mortality in Cushing’s syndrome: systematic analysis of a large series with prolonged follow-up. Eur J Endocrinol. 2013;169(5):715–723. doi: 10.1530/EJE-13-0569. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

