Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome
- PMID: 26507912
- PMCID: PMC4704510
- DOI: 10.1113/JP270966
Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome
Abstract
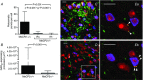
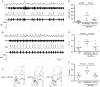
Life threatening breathing irregularity and central apnoeas are highly prevalent in children suffering from Rett syndrome. Abnormalities in inhibitory synaptic transmission have been associated with the physiopathology of this syndrome, and may underlie the respiratory disorder. In a mouse model of Rett syndrome, GABAergic terminal projections are markedly reduced in the Kölliker-Fuse nucleus (KF) in the dorsolateral pons, an important centre for control of respiratory rhythm regularity. Administration of a drug that augments endogenous GABA localized to this region of the pons reduced the incidence of apnoea and the respiratory irregularity of Rett female mice. Conversely, the respiratory disorder was recapitulated by blocking GABAergic transmission in the KF area of healthy rats. This study helps us understand the mechanism for generation of respiratory abnormality in Rett syndrome, pinpoints a brain site responsible and provides a clear anatomical target for the development of a translatable drug treatment. Central apnoeas and respiratory irregularity are a common feature in Rett syndrome (RTT), a neurodevelopmental disorder most often caused by mutations in the methyl-CpG-binding protein 2 gene (MECP2). We used a MECP2 deficient mouse model of RTT as a strategy to obtain insights into the neurobiology of the disease and into mechanisms essential for respiratory rhythmicity during normal breathing. Previously, we showed that, systemic administration of a GABA reuptake blocker in MECP2 deficient mice markedly reduced the occurrence of central apnoeas. Further, we found that, during central apnoeas, post-inspiratory drive (adductor motor) to the upper airways was enhanced in amplitude and duration in Mecp2 heterozygous female mice. Since the pontine Kölliker-Fuse area (KF) drives post-inspiration, suppresses inspiration, and can reset the respiratory oscillator phase, we hypothesized that synaptic inhibition in this area is essential for respiratory rhythm regularity. In this study, we found that: (i) Mecp2 heterozygous mice showed deficiency of GABA perisomatic bouton-like puncta and processes in the KF nucleus; (ii) blockade of GABA reuptake in the KF of RTT mice reduced breathing irregularity; (iii) conversely, blockade of GABAA receptors in the KF of healthy rats mimicked the RTT respiratory phenotype of recurrent central apnoeas and prolonged post-inspiratory activity. Our results show that reductions in synaptic inhibition within the KF induce rhythm irregularity whereas boosting GABA transmission reduces respiratory arrhythmia in a murine model of RTT. Our data suggest that manipulation of synaptic inhibition in KF may be a clinically important strategy for alleviating the life threatening respiratory disorders in RTT.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



References
-
- Alheid GF, Gray PA, Jiang MC, Feldman JL & McCrimmon DR (2002). Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol 31, 693–717. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

