Competition, Selectivity and Efficacy of Analogs of A-84543 for Nicotinic Acetylcholine Receptors with Repositioning of Pyridine Nitrogen
- PMID: 26508288
- PMCID: PMC4741274
- DOI: 10.1007/s11064-015-1705-z
Competition, Selectivity and Efficacy of Analogs of A-84543 for Nicotinic Acetylcholine Receptors with Repositioning of Pyridine Nitrogen
Abstract
Nicotinic acetylcholine receptors (nAChRs) play a crucial role in a number of clinically relevant mental and neurological pathways, as well as autonomic and immune functions. The development of subtype-selective ligands for nAChRs therefore is potentially useful for targeted therapeutic management of conditions where nAChRs are involved. We tested if selectivity for a particular nAChR subtype can be achieved through small structural modifications of a lead compound containing the nicotinic pharmacophore by changing the distance between the electronegative elements. For this purpose, analogs of A-84543 were designed, synthesized and characterized as potentially new nAChR subtype-selective ligands. Compounds were tested for their binding properties in rat cerebral cortical tissue homogenates, and subtype-selectivity was determined using stably transfected HEK cells expressing different nAChR subtypes. All compounds synthesized were found to competitively displace [(3)H]-epibatidine ([(3)H]EB) from the nAChR binding site. Of all the analogues, H-11MNH showed highest affinity for nAChRs compared to a ~ fivefold to tenfold lower affinity of A-84543. All other compounds had affinities >10,000 nM. Both A-84543 and H-11MNH have highest affinity for α2β2 and α4β2 nAChRs and show moderate affinity for β4- and α7-containing receptors. H-11MNH was found to be a full agonist with high potency at α3β4, while A-84543 is a partial agonist with low potency. Based on their unique pharmacological binding properties we suggest that A-84543 and its desmethylpyrrolidine analog can be useful as pharmacological ligands for studying nAChRs if selective pharmacological and/or genetic tools are used to mask the function of other receptors subtypes.
Keywords: A-85380; Cerebral cortex; Epibatidine; H-11MNH; Nicotine; Saturation.
Conflict of interest statement
Conflict of Interest: The authors declare that there is no conflict of interest.
Figures
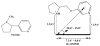



References
-
- Jensen AA, Bente F, Liljefors T, Krogsgard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48(15):4705–4745. - PubMed
-
- Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137(1):22–54. - PubMed
-
- Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

